441090
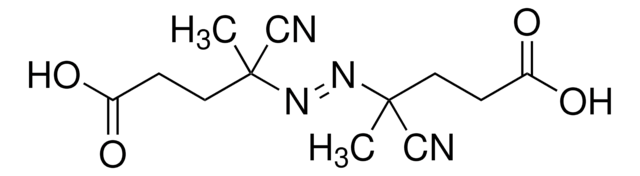
2,2′-Azobis(2-methylpropionitrile)
98%
Synonym(s):
α,α′-Azoisobutyronitrile, AIBN, Azobisisobutyronitrile, Free radical initiator
About This Item
Recommended Products
Quality Level
assay
98%
form
powder
mp
102-104 °C (dec.) (lit.)
storage temp.
2-8°C
SMILES string
CC(C)(\N=N\C(C)(C)C#N)C#N
InChI
1S/C8H12N4/c1-7(2,5-9)11-12-8(3,4)6-10/h1-4H3/b12-11+
InChI key
OZAIFHULBGXAKX-VAWYXSNFSA-N
Looking for similar products? Visit Product Comparison Guide
Application
- Polystyrene by soap-free emulsion polymerization.
- Molecularly imprinted polymer(MIP) using 1-vinyl imidazole. MIP can be used to quantify acid violet 19 dye in river water samples.
Storage and Stability
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Aquatic Chronic 3 - Self-react. C
supp_hazards
Storage Class
4.1A - Other explosive hazardous materials
wgk_germany
WGK 2
flash_point_f
122.0 °F
flash_point_c
50 °C
ppe
dust mask type N95 (US), Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
To keep pace with Moore′s Law, there is a continuing need in the semiconductor industry to achieve higher circuit density in microelectronic devices.
An article regarding common FAQs for initiators and stabilizers.
RAFT polymerization offers living characteristics to radical polymerization, contributing versatility to reversible deactivation radical polymerization methods.
Monomers for ophthalmic use aim for purity, reliability, and comfort, driving innovation for affordable contact lenses.
Protocols
RAFT polymerization offers precise control, enabling tailored synthesis of complex polymer structures.
We present an article about RAFT, or Reversible Addition/Fragmentation Chain Transfer, which is a form of living radical polymerization.
Polymerization via ATRP procedures demonstrated by Prof. Dave Haddleton's research group at the University of Warwick.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service