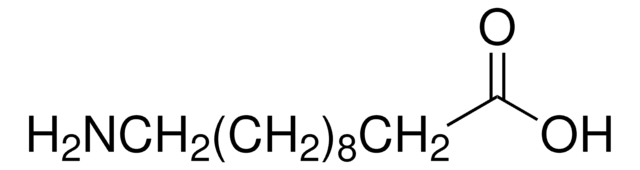159247
12-Aminododecanoic acid
95%
Synonym(s):
12-Aminolauric acid
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
NH2(CH2)11COOH
CAS Number:
Molecular Weight:
215.33
Beilstein/REAXYS Number:
907502
EC Number:
MDL number:
UNSPSC Code:
12352209
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
95%
form
powder or crystals
reaction suitability
reaction type: solution phase peptide synthesis
color
white to off-white
mp
185-187 °C (lit.)
application(s)
peptide synthesis
SMILES string
NCCCCCCCCCCCC(O)=O
InChI
1S/C12H25NO2/c13-11-9-7-5-3-1-2-4-6-8-10-12(14)15/h1-11,13H2,(H,14,15)
InChI key
PBLZLIFKVPJDCO-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
536.0 °F - closed cup
flash_point_c
280 °C - closed cup
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Yasuhisa Fukuta et al.
Bioscience, biotechnology, and biochemistry, 73(5), 980-986 (2009-05-08)
The genes encoding omega-laurolactam hydrolases from Cupriavidus sp. T7, Acidovorax sp. T31, Cupriavidus sp. U124, and Sphingomonas sp. U238 were cloned and sequenced. Nucleotide and amino acid sequence analysis of the four genes indicated that the primary structures of these
Liang Chen et al.
Nanotechnology, 22(10), 105708-105708 (2011-02-04)
The objective of this study is to evaluate the effect of hydroxyapatite (HAP) nanoparticles with different surface charges on the cellular uptake behavior and in vitro cell viability and proliferation of MC3T3-E1 cell lines (osteoblast). The nanoparticles' surface charge was
Yasuhisa Asano et al.
Bioscience, biotechnology, and biochemistry, 72(8), 2141-2150 (2008-08-08)
Several omega-laurolactam degrading microorganisms were isolated from soil samples. These strains were capable of growing in a medium containing omega-laurolactam as sole source of carbon and nitrogen. Among them, five strains (T7, T31, U124, U224, and U238) were identified as
Hiroto Hayashi et al.
International journal of molecular sciences, 12(9), 5490-5507 (2011-10-22)
Novel poly(ester-urethane)s were prepared by a synthetic route using a lipase that avoids the use of hazardous diisocyanate. The urethane linkage was formed by the reaction of phenyl carbonate with amino acids and amino alcohols that produced urethane-containing diacids and
K Kamio et al.
Journal of lipid research, 33(8), 1227-1232 (1992-08-01)
For preparation of an affinity ligand, an N-fatty acyl moiety of galactosylceramide (GalCer) was chemically replaced with omega-amino-fatty acid including amino-n-hexanoic acid or amino-n-dodecanoic acid to obtain omega-aminoGalCer. For the synthesis of the compound, galactosylsphingosine (GalSph) was coupled with N-trifluoroacetyl
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service