SML3616
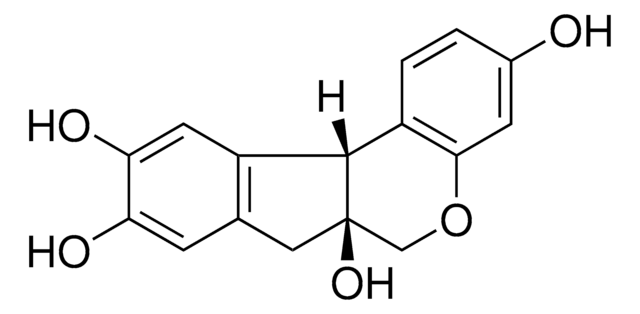
Kazinol U
≥95% (HPLC)
Synonym(s):
(+)-Kazinol U, 4-[(2S)-3,4-Dihydro-7-hydroxy-2H-1-benzopyran-2-yl]-3-(3-methyl-2-buten-1-yl)-1,2-benzenediol
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C20H22O4
CAS Number:
Molecular Weight:
326.39
MDL number:
UNSPSC Code:
12352200
NACRES:
NA.21
Recommended Products
Quality Level
assay
≥95% (HPLC)
form
powder
color
white to beige
solubility
DMSO: 2 mg/mL, clear
storage temp.
-10 to -25°C
Biochem/physiol Actions
Anti-melanogenic in mouse and human melanoma cells through the induction of AMPK and MAPK phosphorylation
Kazinol U is prenylated flavan isolated from an extract of Broussonetia kazinoki Sieb (paper mulberry) that exhibits anti-melanogenic activity in mouse and human melanoma cells through the induction of AMPK and MAPK phosphorylation. Kazinol U inhibits tyrosinase activity and melanin synthesis through the reduction of MITF expression. It suppresses melanogenesis in a zebrafish model. Kazinol U displays phytoestrogenic and anti-inflammatory activities. Kazinol U exhibits cytoprotective activities against cytokine-induced apoptotic cell death.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Jihyun Lim et al.
British journal of pharmacology, 176(5), 737-750 (2018-12-24)
Kazinol U is a prenylated flavan isolated from an extract of Broussonetia kazinoki Sieb (Moraceae). Kazinol U has shown cytoprotective effects against cytokine-induced apoptotic cell death and induces AMP kinase (AMPK) activation through LKB1 activation. However, kazinol U has not
Ui-Jin Bae et al.
Biological & pharmaceutical bulletin, 34(7), 1026-1031 (2011-07-02)
The generation of nitric oxide (NO) via inducible NO synthase (iNOS) and reactive oxygen species plays a key role in cytokine-mediated pancreatic β-cell damage. Oxidative stress due to reactive oxygen species activates the nuclear factor-κB (NF-κB) transcription factor, which regulates
Qian Sun et al.
European journal of medicinal chemistry, 133, 1-10 (2017-04-04)
Enantiomers account for quite a large percentage of compounds in natural products. Our team is interested in the separation and biological activity of racemic compounds. In this report, four pairs of prenylated flavan enantiomers [(±)-1-(±)-4], including five new compounds, were
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service