L2884
Leupeptin
powder, ≥90% (HPLC)
Synonym(s):
Leupeptin hemisulfate salt, Acetyl-Leu-Leu-Arg-al, N-Acetyl-L-leucyl-L-leucyl-L-argininal hemisulfate salt
About This Item
Recommended Products
product name
Leupeptin, microbial, ≥90% (HPLC)
biological source
microbial
Quality Level
assay
≥90% (HPLC)
form
powder
solubility
H2O: 10 mM (Solutions are stable for a week at 4 °C. Stock solutions are stable up to 6 months at −20 °C.)
H2O: 50 mg/mL
storage temp.
−20°C
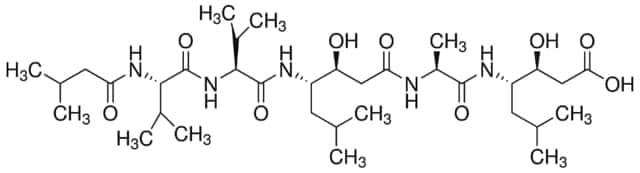
SMILES string
OS(O)(=O)=O.CC(C)C[C@H](NC(C)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C=O.CC(C)C[C@H](NC(C)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C=O
InChI
1S/2C20H38N6O4.H2O4S/c2*1-12(2)9-16(24-14(5)28)19(30)26-17(10-13(3)4)18(29)25-15(11-27)7-6-8-23-20(21)22;1-5(2,3)4/h2*11-13,15-17H,6-10H2,1-5H3,(H,24,28)(H,25,29)(H,26,30)(H4,21,22,23);(H2,1,2,3,4)/t2*15-,16-,17-;/m00./s1
InChI key
CIPMKIHUGVGQTG-VFFZMTJFSA-N
Looking for similar products? Visit Product Comparison Guide
General description
- In the active site of serine proteases, leupeptin forms a covalent hemiacetal adduct between the aldehyde group of leupeptin and the hydroxyl group of a serine residue in the enzyme active site.
- In the active site of cysteine proteases, the electrophilic (aldehyde) carbon of leupeptin forms a comparable bond with the sulfur atom of a cysteine residue in the enzyme active site.
Biochem/physiol Actions
Analysis Note
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
ReadyShield® phosphatase and protease inhibitor cocktail FAQ for sample protection in a variety of cell types and tissue extracts, including mammalian, plant, and microbial samples. Our ReadyShield® Protease Inhibitor Cocktail is a non-freezing solution that contains inhibitors with a broad specificity for serine, cysteine, acid proteases and aminopeptidases.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service