F9302
Factor X Activated (Xa) from bovine plasma
aqueous glycerol solution
Synonym(s):
Factor Xa
Sign Into View Organizational & Contract Pricing
All Photos(4)
About This Item
CAS Number:
MDL number:
UNSPSC Code:
12352204
NACRES:
NA.54
Recommended Products
form
aqueous glycerol solution
Quality Level
specific activity
≥50 units/mg protein
UniProt accession no.
shipped in
wet ice
storage temp.
−20°C
Gene Information
cow ... F10(280787)
Looking for similar products? Visit Product Comparison Guide
Application
Fusion proteins are commonly expressed with a factor Xa cleavable Ile-Glu (or Asp)-Gly-Arg-↓-X sequence. Typically 1 mg of fusion protein can be incubated with 10 μg of factor Xa for 2.5 hours at 37 °C.
Biochem/physiol Actions
Factor Xa catalyzes the hydrolysis of the Arg-Thr and then Arg-Ile bonds in prothrombin to yield active thrombin.
The fairly strict recognition sequence is Ile-Glu (or Asp)-Gly-Arg-↓-X.
It may sometimes cleave at other basic residues, depending on the conformation of the target protein. Factor Xa will not cleave if a proline residue follows the arginine of the recognition sequence.
pH Optimum: 7.6-8.0
Temperature Optimum: 37 °C
The fairly strict recognition sequence is Ile-Glu (or Asp)-Gly-Arg-↓-X.
It may sometimes cleave at other basic residues, depending on the conformation of the target protein. Factor Xa will not cleave if a proline residue follows the arginine of the recognition sequence.
pH Optimum: 7.6-8.0
Temperature Optimum: 37 °C
Physical properties
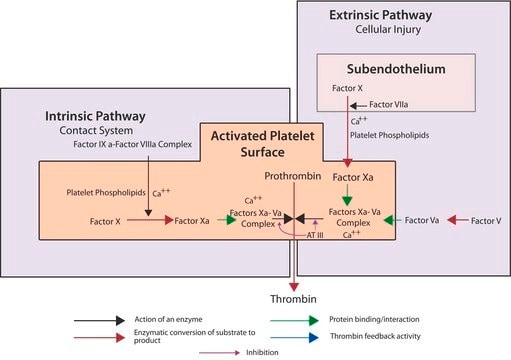
Factor Xa is a serine endoproteinase and a member of the S1 peptidase family. Factor Xa plays a critical role in the coagulation cascade by catalyzing the proteolytic conversion of prothrombin to active thrombin. Factor Xa′s prothrombin conversion activity is greatly enhanced in vivo when complexed with factor V, calcium ions and phospholipids on the activated platelet surface.
The zymogen form, Factor X, is activated in vivo by two different pathways. The intrinsic pathway utilizes a catalytic complex composed of factor IXa, factor VIII, phospholipids and calcium ions. The extrinsic pathway utilizes a complex of factor VII and tissue factor. The factor X zymogen is a 55 KDa glycoprotein with a light and heavy chain joined by a single disulfide.
The zymogen form, Factor X, is activated in vivo by two different pathways. The intrinsic pathway utilizes a catalytic complex composed of factor IXa, factor VIII, phospholipids and calcium ions. The extrinsic pathway utilizes a complex of factor VII and tissue factor. The factor X zymogen is a 55 KDa glycoprotein with a light and heavy chain joined by a single disulfide.
Unit Definition
One unit of activated Factor X will liberate 1.0 μmole of p-nitroanilide from N-benzoyl-L-isoleucyl-L-glutamyl-L-glycyl-L-arginine-p-nitroaniline per minute at pH 8.3 at 37 °C.
Disclaimer
RESEARCH USE ONLY. This product is regulated in France when intended to be used for scientific purposes, including for import and export activities (Article L 1211-1 paragraph 2 of the Public Health Code). The purchaser (i.e. enduser) is required to obtain an import authorization from the France Ministry of Research referred in the Article L1245-5-1 II. of Public Health Code. By ordering this product, you are confirming that you have obtained the proper import authorization.
Substrate
Product No.
Description
Pricing
Storage Class
10 - Combustible liquids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Tyan F Thomas et al.
Clinical therapeutics, 35(1), 4-27 (2013-01-19)
Currently available anticoagulants utilized for venous thromboembolism (VTE) treatment and prevention and stroke prevention in patients with atrial fibrillation (AF) have proven effectiveness but are not optimally utilized because of barriers such as the need for subcutaneous administration and requisite
Is Apixaban (Eliquis) the "ideal" anticoagulant to replace warfarin for stroke prevention in atrial fibrillation?
Joe Strain et al.
South Dakota medicine : the journal of the South Dakota State Medical Association, 66(1), 20-21 (2013-01-25)
Alpesh Amin
Clinical interventions in aging, 8, 75-84 (2013-02-05)
Atrial fibrillation (AF) is associated with an increased incidence and severity of strokes. The burden of AF-related stroke is expected to increase in parallel with the aging of the population. Oral anticoagulation with warfarin has been the pharmacologic standard for
Manesh R Patel et al.
Journal of the American College of Cardiology, 61(6), 651-658 (2013-02-09)
The purpose of this study was to understand the possible risk of discontinuation in the context of clinical care. Rivaroxaban is noninferior to warfarin for preventing stroke in atrial fibrillation patients. Concerns exist regarding possible increased risk of stroke and
Jeremy W Vandiver et al.
Hospital practice (1995), 41(2), 16-24 (2013-04-03)
To determine if laboratory monitoring of intravenous (IV) unfractionated heparin (UFH) using an anti–activated factor X (anti–factor Xa) assay, as opposed to the activated partial thromboplastin time (aPTT), would result in a higher percentage of results within the goal range
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service