C6286
Cathepsin B from bovine spleen
lyophilized powder, ≥10 units/mg protein
Synonym(s):
Cathepsin B1
Sign Into View Organizational & Contract Pricing
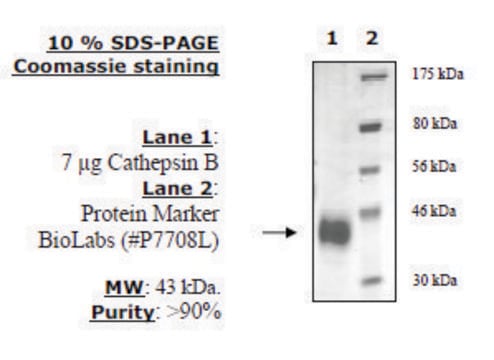
All Photos(1)
About This Item
CAS Number:
MDL number:
UNSPSC Code:
12352204
NACRES:
NA.32
Recommended Products
biological source
bovine spleen
form
lyophilized powder
specific activity
≥10 units/mg protein
composition
Protein, ≥30% biuret
UniProt accession no.
storage temp.
−20°C
Gene Information
cow ... CTSB(281105)
Looking for similar products? Visit Product Comparison Guide
General description
Cathepsin B is a lysosomal enzyme that comprises a light chain (Lys1–Arg49) and a heavy chain (Val50–Thr253). It belongs to cysteine family C1.
Application
Cathepsin B from bovine spleen has been used in cleavage assay before high-performance liquid chromatography-mass spectrometry (HPLC-MS) analysis. It has also been used in the enzyme inhibition assays with protease inhibitors RfIP1 and ruthenium metalloarenes.
Biochem/physiol Actions
Cathepsin B displays an endopeptidase and peptidyl dipeptidase activities. It may be associated with the pathophysiology of tumors. Cathepsin B is also regarded as a prominent protease in Leishmaniasis. It is overactivated in muscular dystrophy, pulmonary emphysema, and bone resorption.
Cathepsin B has been found to cleave procaspase 1 and procaspase 11 and to induce apoptosis in digitonin-permeabilized cells. Translocation of cathepsin B from the cytoplasm to the nucleus contributes to bile salt induced apoptosis of rat hepatocytes. Levels of cathepsin B in PC12 cells significantly decrease 12 to 24 hours after apoptosis is induced.
Unit Definition
One unit will hydrolyze 1 μmole of Z-lysine p-nitrophenyl ester per min at pH 5.0 at 25 °C.
Physical form
Lyophilized powder containing sodium phosphate, sodium chloride and ~6% EDTA as stabilizer.
inhibitor
Product No.
Description
Pricing
related product
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Angela Casini et al.
Dalton transactions (Cambridge, England : 2003), 39(23), 5556-5563 (2010-05-15)
A series of organometallic compounds of general formula [(arene)M(PTA)(n)X(m)]Y (arene = eta(6)-C(10)H(14), eta-C(5)Me(5)); M = Ru(ii), Os(ii), Rh(iii) and Ir(iii); X = Cl, mPTA; Y = OTf, PF(6)) have been screened for their cytotoxicity and ability to inhibit cathepsin B
Angela Casini et al.
Journal of medicinal chemistry, 51(21), 6773-6781 (2008-10-07)
A series of ruthenium(II)-arene (RAPTA) compounds were evaluated for their ability to inhibit thioredoxin reductase (either cytosolic or mitochondrial) and cathepsin B, two possible targets for anticancer metallodrugs. In general, inhibition of the thioredoxin reductases was lower than that of
Abir Ben Bacha et al.
3 Biotech, 7(2), 148-148 (2017-06-10)
Protease inhibitors from plants are well known to be potent inhibitors of the growth of bacteria, fungi, and even certain viruses which make them excellent candidates for use as the lead compounds for the development of novel antimicrobial agents for
M A Sentandreu et al.
Biochemistry and cell biology = Biochimie et biologie cellulaire, 81(4), 317-326 (2003-10-22)
Cathepsin B (EC 3.4.22.1) has been highly purified (14,225 fold) from bovine kidney by a rapid procedure that included the preparation of an enriched lysosomal extract, a selective fractionation with ammonium sulphate, size-exclusion chromatography, two cation-exchange chromatographies, and anion-exchange chromatography
Pasquale Mura et al.
Journal of inorganic biochemistry, 104(2), 111-117 (2009-11-27)
A novel ruthenium(II) compound, trans-cis-cis-[Ru(II)Cl(2)(DMSO)(2)(2-amino-5-methyl-thiazole)(2)], (I), PMRu52 hereafter, that may be obtained from the previously described (cis and trans)-[Ru(II)Cl(2)(DMSO)(4)] complexes, was designed, synthesized and characterised. The single crystal X-ray structure shows a roughly regular octahedral environment for the ruthenium(II) center
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service