A2385
5-Azacytidine
≥98% (HPLC), powder, DNA methyltransferase inhibitor
Synonym(s):
5-Azacitidine, 4-Amino-1-(β-D-ribofuranosyl)-1,3,5-triazin-2(1H)-one, Ladakamycin
About This Item
Recommended Products
product name
5-Azacytidine, ≥98% (HPLC)
assay
≥98% (HPLC)
form
powder
mp
226-232 °C (dec.) (lit.)
antibiotic activity spectrum
viruses
mode of action
DNA synthesis | interferes
originator
Celgene
storage temp.
−20°C
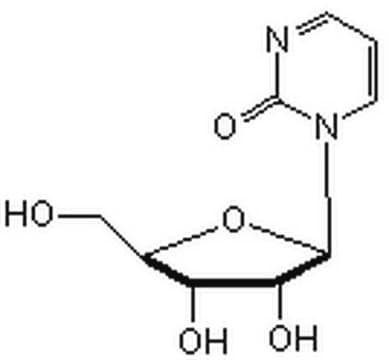
SMILES string
NC1=NC(=O)N(C=N1)[C@@H]2O[C@H](CO)[C@@H](O)[C@H]2O
InChI
1S/C8H12N4O5/c9-7-10-2-12(8(16)11-7)6-5(15)4(14)3(1-13)17-6/h2-6,13-15H,1H2,(H2,9,11,16)/t3-,4-,5-,6-/m1/s1
InChI key
NMUSYJAQQFHJEW-KVTDHHQDSA-N
Gene Information
human ... DNMT1(1786) , DNMT3A(1788) , DNMT3B(1789)
Looking for similar products? Visit Product Comparison Guide
General description
Application
- for cell induction
- to study its effects on bovine fetal mesenchymal stem cells (bfMSC)
- to study its effects on human bone marrow stromal cells (hBMSCs)
- to study its effects on and DNA methyltransferase 1 (DNMT1) and Ras protein activator like 1 (RASAL1) expression
- to interfere with DNA methylation and histone acetylation
- to determine its effects on the conversion of control fibroblasts
- for optical coherence tomography (OCT) and fluorescein angiography (FA)
- for the reactivation of Sal-like protein (SALL)3 expression
Biochem/physiol Actions
Features and Benefits
signalword
Danger
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Chronic 1 - Carc. 1A - Muta. 2 - Repr. 1B - STOT RE 1
Storage Class
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
wgk_germany
WGK 3
ppe
Eyeshields, Gloves, type P3 (EN 143) respirator cartridges
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Cancer research has revealed that the classical model of carcinogenesis, a three step process consisting of initiation, promotion, and progression, is not complete.
We offer a variety of small molecule research tools, such as transcription factor modulators, inhibitors of chromatin modifying enzymes, and agonists/antagonists for target identification and validation in gene regulation research; a selection of these research tools is shown below.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service












![Imidazo[1,2-a]pyridine-7-carboxylic acid AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/307/298/b27c0495-0260-443a-9af3-d106cfb691a6/640/b27c0495-0260-443a-9af3-d106cfb691a6.png)