All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C13H10O
CAS Number:
Molecular Weight:
182.22
Beilstein/REAXYS Number:
133939
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
Recommended Products
assay
99%
bp
310-312 °C (lit.)
mp
101-102 °C (lit.)
SMILES string
C1c2ccccc2Oc3ccccc13
InChI
1S/C13H10O/c1-3-7-12-10(5-1)9-11-6-2-4-8-13(11)14-12/h1-8H,9H2
InChI key
GJCOSYZMQJWQCA-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Xanthene (10H-9-oxaanthracene) is a heterocyclic compound generally used as an important building block in organic synthesis. It is a core moiety of many dyes like fluorescein, eosins, and rhodamines.
Application
Xanthene can be used as:
- A reactant in the dehydrogenative cross-coupling reaction with carbonyl compounds.
- A substrate to synthesize N-xanthyl-p-toluenesulfonamide via C−H amination with tosyl azide using rhodium catalyst.
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Copper-catalyzed aerobic asymmetric cross-dehydrogenative coupling of C (sp3)-H bonds driven by visible light
Zhou K, et al.
Green Chemistry, 22(14), 4597-4603 (2020)
Meng Chen et al.
Journal of chromatography. A, 1590, 27-38 (2019-01-12)
An analytical methodology for comprehensive screening of 63 coloring agents of great concern for regulatory control in cosmetics has been established using ultra-high-performance liquid chromatography (UHPLC) coupled with quadrupole-Orbitrap high-resolution mass spectrometry (Q-Orbitrap HRMS). An effective, rapid, and simple sample
Evgeniy Dukhopelnykov et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 247, 119114-119114 (2020-11-10)
The interaction between xanthene dye eosin Y and double stranded DNA has been studied by spectrophotometry. The conventional titration study does not show the interaction in the eosin Y - DNA system. Therefore, the competitive binding assay was carried out.
Yita Wang et al.
Nanomaterials (Basel, Switzerland), 8(7) (2018-07-07)
The electro-Fenton (EF) process is a type of electrochemical oxidation process; ·OH radicals are generated on the cathode using electricity and decolorize dye wastewaters. Most studies on EF systems in the past have focused on the operating parameters of this
Electrochemical dehydrogenative cross-coupling of xanthenes with ketones
Yang Y-Z, et al.
Chemical Communications (Cambridge, England), 56(14), 7585-7588 (2020)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service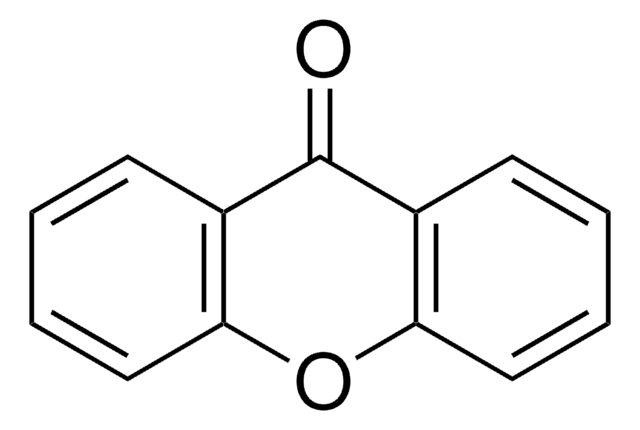











![2-(5-Bromo-2-pyridylazo)-5-[N-propyl-N-(3-sulfopropyl)amino]phenol disodium salt dihydrate ≥95.0% (HPLC), for spectrophotometric det. of Zn(II), Cu(II), Fe(II), Co(II), H2O2](/deepweb/assets/sigmaaldrich/product/structures/156/597/5c8f3945-13eb-4a6f-97b9-80540225a5c1/640/5c8f3945-13eb-4a6f-97b9-80540225a5c1.png)
