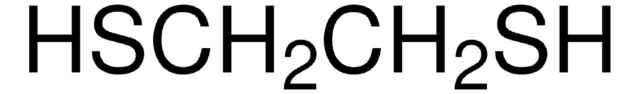W349518
1,6-Hexanedithiol
≥97%, FG
Synonym(s):
1,6-Dimercaptohexane, DMH, Hexamethylene dimercaptan
About This Item
Recommended Products
biological source
synthetic
Quality Level
grade
FG
Halal
Kosher
reg. compliance
EU Regulation 1334/2008 & 178/2002
vapor pressure
~1 mmHg ( 20 °C)
assay
≥97%
refractive index
n20/D 1.511 (lit.)
bp
118-119 °C/15 mmHg (lit.)
mp
−21 °C (lit.)
density
0.983 g/mL at 25 °C (lit.)
application(s)
flavors and fragrances
documentation
see Safety & Documentation for available documents
food allergen
no known allergens
organoleptic
burnt; fatty; meaty
SMILES string
SCCCCCCS
InChI
1S/C6H14S2/c7-5-3-1-2-4-6-8/h7-8H,1-6H2
InChI key
SRZXCOWFGPICGA-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
- Magnetoresistance originated from the Au/S interface in Au/1,6-hexanedithiol/Au single-molecule junctions at room temperature.: This study demonstrates the potential of 1,6-hexanedithiol in creating single-molecule junctions that exhibit magnetoresistance at room temperature. Such findings could revolutionize the development of molecular electronics by enhancing the functional properties of these junctions for more efficient and scalable applications (Andika et al., 2023).
- Sensitivity investigation of a biosensor with resonant coupling of propagating surface plasmons to localized surface plasmons in the near infrared region.: This research highlights the use of 1,6-hexanedithiol in enhancing the sensitivity of plasmonic biosensors. The thiol′s ability to bridge nanoparticles enhances surface plasmon resonance, critical for developing highly sensitive diagnostic tools (Wang et al., 2023).
- Facile Synthesis of Light-Switchable Polymers with Diazocine Units in the Main Chain.: In this innovative work, 1,6-hexanedithiol is employed to synthesize light-switchable polymers, showcasing its utility in creating responsive materials. These polymers have potential applications in smart coatings and adaptive materials, adjusting properties in response to environmental stimuli (Li et al., 2023).
- Conjugation monitoring of gold nanoparticles with alkanedithiols by capillary zone electrophoresis.: This study utilizes 1,6-hexanedithiol for the conjugation of gold nanoparticles, illustrating its effectiveness in facilitating stable and reproducible nanoconjugates. Such capabilities are essential for the advancement of nanotechnology applications in medicine and materials science (Takayanagi et al., 2023).
- Enhanced charge transport across molecule-nanoparticle-molecule sandwiches.: Research demonstrates that 1,6-hexanedithiol can significantly enhance charge transport in nanoparticle assemblies, suggesting its role in developing more efficient energy transfer systems for electronic devices and solar cells (Zhou et al., 2023).
Storage Class
10 - Combustible liquids
wgk_germany
WGK 3
flash_point_f
195.8 °F - closed cup
flash_point_c
91 °C - closed cup
ppe
Eyeshields, Gloves, multi-purpose combination respirator cartridge (US)
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service