T33200
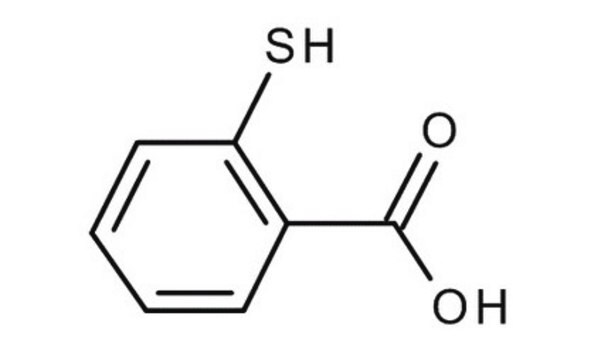
Thiosalicylic acid
97%
Synonym(s):
2-Sulfanylbenzoic acid, 2-Mercaptobenzoic acid
Sign Into View Organizational & Contract Pricing
All Photos(4)
About This Item
Linear Formula:
HSC6H4CO2H
CAS Number:
Molecular Weight:
154.19
Beilstein/REAXYS Number:
508507
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
97%
mp
162-165 °C (lit.)
SMILES string
OC(=O)c1ccccc1S
InChI
1S/C7H6O2S/c8-7(9)5-3-1-2-4-6(5)10/h1-4,10H,(H,8,9)
InChI key
NBOMNTLFRHMDEZ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Thiosalicylic acid can be used as:
It can also be used to prepare 2-thioxanthone-thioacetic acid bimolecular system, which is used as a photoinitiator for free radical polymerization.
- A nucleophilic trapping agent for the desulfenylation of 3-indolyl sulfides to obtain 3-unsubstituted indoles.
- A starting material to prepare 2′-mercaptoacetophenone, which is used in the synthesis of thioflavanone by reacting with lithium diisopropylamide and benzaldehyde.
- A stabilizing agent in the synthesis of metal nanoparticles.
It can also be used to prepare 2-thioxanthone-thioacetic acid bimolecular system, which is used as a photoinitiator for free radical polymerization.
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
A K Singh et al.
Journal of chromatography, 568(2), 351-361 (1991-08-23)
Simple and reproducible high-performance liquid chromatographic (HPLC) and gas chromatographic-mass spectrometric (GC-MS) methods have been developed for the simultaneous analysis of several acidic drugs in horse plasma and urine. Although the capillary GC-MS column provided better separation of the drugs
A new synthesis of thioflavanones from thiosalicylic acid
Lee Jae-In
Bulletin of the Korean Chemical Society,, 29(6), 1263-1265 (2008)
R Grossman et al.
NeuroImage, 20(4), 1971-1981 (2003-12-20)
Increases in peripheral type benzodiazepine receptors (PTBR) have been utilized for the detection of neuroinflammation and neurotoxicity in the brain. We have investigated the relationship between PTBR and NMDA receptor binding density in mice with closed head injury (CHI) using
Synthesis, 309-309 (1994)
S Chong et al.
Biochemical pharmacology, 42(7), 1433-1439 (1991-09-12)
Co-administration of N-acetylcysteine (NAC) with nitroglycerin (NTG) has been shown to partially reverse nitrate tolerance and to potentiate the hypotensive effect of NTG in humans. However, a high clinical dose of NAC was required for this pharmacologic interaction resulting in
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service