P35200
Phenylsuccinic acid
98%
Synonym(s):
(±)-Phenylsuccinic acid
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
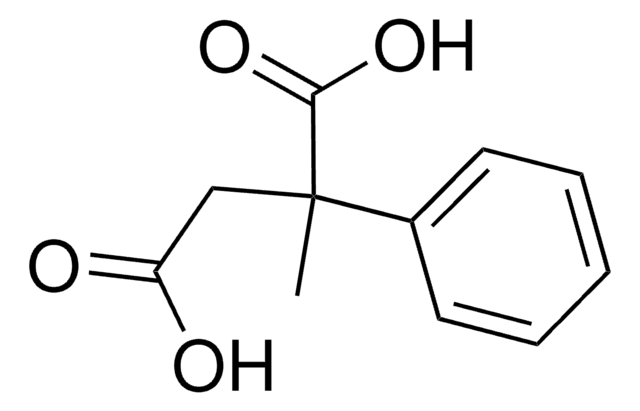
HO2CCH2CH(C6H5)CO2H
CAS Number:
Molecular Weight:
194.18
Beilstein/REAXYS Number:
1875051
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
98%
form
powder
mp
166-168 °C (lit.)
SMILES string
OC(=O)CC(C(O)=O)c1ccccc1
InChI
1S/C10H10O4/c11-9(12)6-8(10(13)14)7-4-2-1-3-5-7/h1-5,8H,6H2,(H,11,12)(H,13,14)
InChI key
LVFFZQQWIZURIO-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
R Huang et al.
Neuroscience letters, 183(1-2), 22-26 (1995-01-02)
Phenylsuccinate is an inhibitor of cytosolic glutamate formation from extracellular glutamine in cultured cerebellar granule cell neurons, a glutamatergic preparation. It prevents anoxic cell death in these cells as indicated by decreased lactate dehydrogenase (LDH) release and by the morphological
Heather M Wilkins et al.
The Journal of biological chemistry, 288(7), 5091-5101 (2013-01-04)
Mitochondrial oxidative stress significantly contributes to the underlying pathology of several devastating neurodegenerative disorders. Mitochondria are highly sensitive to the damaging effects of reactive oxygen and nitrogen species; therefore, these organelles are equipped with a number of free radical scavenging
A Atlante et al.
FEBS letters, 396(2-3), 279-284 (1996-11-04)
In this study we have investigated hydroxyproline transport in rat heart mitochondria and, in particular, in heart left ventricle mitochondria isolated from both spontaneously hypertensive and Wistar-Kyoto rats. Hydroxyproline uptake by mitochondria, where its catabolism takes place, occurs via a
R Svarna et al.
Neurochemical research, 21(5), 603-608 (1996-05-01)
The effect of aminooxyacetic acid (AOAA), NH4+, phenylsuccinate (Phs), ketone bodies (KB) and glutamine (Gln), that might interfere with the biosynthesis of neurotransmitter glutamate on the K(+)-evoked Ca(2+)-dependent release of D-[3H]aspartate from rat cerebellar slices was studied. Therefore slices were
G Palaiologos et al.
Neurochemical research, 14(4), 359-366 (1989-04-01)
Evoked release of glutamate and aspartate from cultured cerebellar granule cells was studied after preincubation of the cells in tissue culture medium with glucose (6.5 mM), glutamine (1.0 mM), D[3H] aspartate and in some cases aminooxyacetate (5.0 mM) or phenylsuccinate
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service