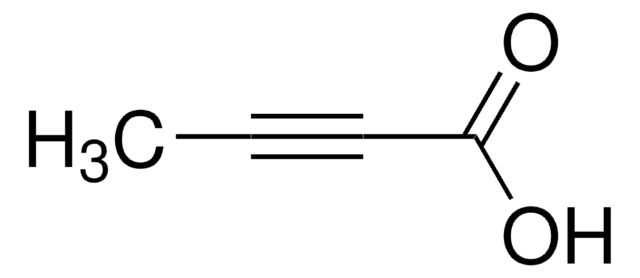P31205
Phenylpropiolic acid
99%
Synonym(s):
Phenylpropynoic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C6H5C≡CCOOH
CAS Number:
Molecular Weight:
146.14
Beilstein/REAXYS Number:
742587
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
99%
form
crystals
mp
135-137 °C (lit.)
storage temp.
2-8°C
SMILES string
OC(=O)C#Cc1ccccc1
InChI
1S/C9H6O2/c10-9(11)7-6-8-4-2-1-3-5-8/h1-5H,(H,10,11)
InChI key
XNERWVPQCYSMLC-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Phenylpropiolic acid can:
- React with 2-tert-butoxypyridine in the presence of boron trifluoride·diethyl etherate to form the corresponding tert-butyl ester.
- Undergo decarboxylative coupling with aryl halides such as p-chloroiodobenzene and 1-chloro-4-iodobenzene.
- Undergo halodecarboxylation to form 1-haloalkynes.
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Neil R McIntyre et al.
Journal of enzyme inhibition and medicinal chemistry, 31(4), 551-562 (2015-05-30)
Peptidylglycine α-amidating monooxygenase (PAM) is a bifunctional enzyme that catalyzes the final reaction in the maturation of α-amidated peptide hormones. Peptidylglycine α-hydroxylating monooxygenase (PHM) is the PAM domain responsible for the copper-, ascorbate- and O2-dependent hydroxylation of a glycine-extended peptide.
M Zieliński et al.
Isotopes in environmental and health studies, 37(3), 239-252 (2002-04-02)
13C kinetic isotope effect (KIE) in the decarboxylation of phenylpropiolic acid (PPA) in tetralin medium (Tn) has been determined at 409-432 K and found to be of magnitude similar to the 13C KIE observed in the decarboxylation of malonic acid
Jill Wood et al.
Bioorganic & medicinal chemistry letters, 16(18), 4965-4968 (2006-06-30)
A series of 4,5-disubstituted cis-pyrrolidinones was investigated as inhibitors of 17beta-HSD II for the treatment of osteoporosis. Biochemical data for several compounds are given. Compound 42 was selected as the lead candidate.
K N Kim et al.
The Journal of prosthetic dentistry, 67(6), 794-798 (1992-06-01)
The viscosity of monophase addition silicone impression materials was measured as a function of shear rate. The setting of mixed catalyst and base was prevented by addition of a small amount of phenyl propiolic acid. All products showed a 6-
Jan Schwarzbauer et al.
Water research, 39(19), 4735-4748 (2005-11-11)
Detailed gas chromatographic-mass spectrometric analyses applied to eight Rhine river water samples constituted a comprehensive characterization of the low molecular weight organic contamination. Within the group of predominant anthropogenic contaminants, only a few compounds were characterized as frequently detected or
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service