N15553
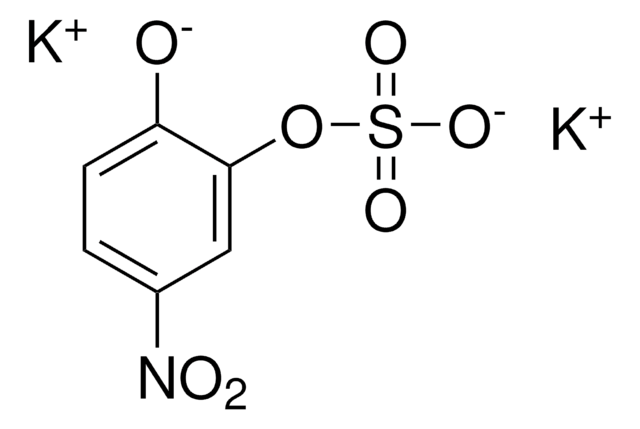
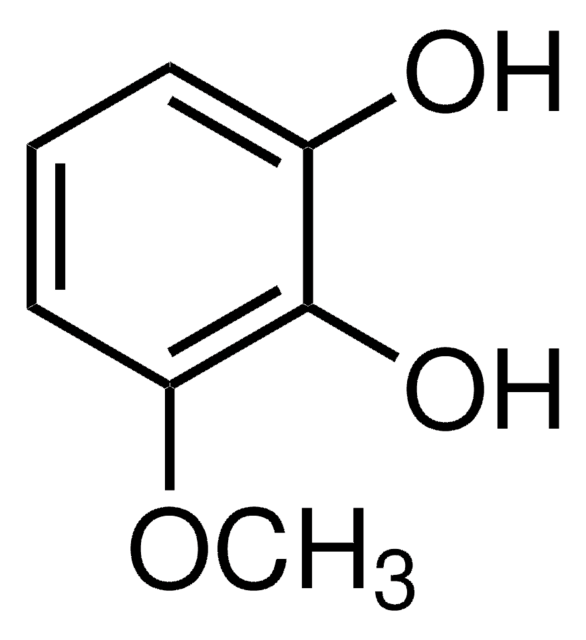
4-Nitrocatechol
≥96.0%
Synonym(s):
1,2-Dihydroxy-4-nitrobenzene, 4-Nitropyrocatechol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
O2NC6H3-1,2-(OH)2
CAS Number:
Molecular Weight:
155.11
Beilstein/REAXYS Number:
1867508
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
≥96.0%
form
powder
mp
173-177 °C (lit.)
SMILES string
Oc1ccc(cc1O)[N+]([O-])=O
InChI
1S/C6H5NO4/c8-5-2-1-4(7(10)11)3-6(5)9/h1-3,8-9H
InChI key
XJNPNXSISMKQEX-UHFFFAOYSA-N
Gene Information
rat ... Nos1(24598)
Looking for similar products? Visit Product Comparison Guide
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Ayelet Fishman et al.
Biotechnology and bioengineering, 87(6), 779-790 (2004-08-27)
After discovering that toluene 4-monooxygenase (T4MO) of Pseudomonas mendocina KR1 oxidizes nitrobenzene to 4-nitrocatechol, albeit at a very low rate, this reaction was improved using directed evolution and saturation mutagenesis. Screening 550 colonies from a random mutagenesis library generated by
A Chauhan et al.
Journal of applied microbiology, 88(5), 764-772 (2000-05-03)
Pseudomonas cepacia RKJ200 (now described as Burkholderia cepacia) has been shown to utilize p-nitrophenol (PNP) as sole carbon and energy source. The present work demonstrates that RKJ200 utilizes 4-nitrocatechol (NC) as the sole source of carbon, nitrogen and energy, and
R J Duescher et al.
Analytical biochemistry, 212(2), 311-314 (1993-08-01)
p-Nitrophenol hydroxylation to p-nitrocatechol is a useful metabolic marker for the presence of functional cytochrome P450 2E1 in mammalian cell microsomes, but the assay is limited by the sensitivity of the spectrophotometric method used to monitor p-nitrocatechol formation. In this
Mark F Reynolds et al.
Journal of biological inorganic chemistry : JBIC : a publication of the Society of Biological Inorganic Chemistry, 8(3), 263-272 (2003-02-18)
Mn(II)-dependent 3,4-dihydroxyphenylacetate 2,3-dioxygenase (MndD) is an extradiol-cleaving catechol dioxygenase from Arthrobacter globiformis that has 82% sequence identity to and cleaves the same substrate (3,4-dihydroxyphenylacetic acid) as Fe(II)-dependent 3,4-dihydroxyphenylacetate 2,3-dioxygenase (HPCD) from Brevibacterium fuscum. We have observed that MndD binds the
Yongjun Liu et al.
Journal of hazardous materials, 181(1-3), 1010-1015 (2010-06-26)
Liquid-phase decomposition of 4-nitrophenol (4-NP) and formation of hydrogen peroxide (H(2)O(2)) induced by contact glow discharge electrolysis (CGDE) were investigated. Experimental results showed that the decays of 4-NP and total organic carbon (TOC) obeyed the first-order and pseudo-first-order reaction kinetics
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service