M86804
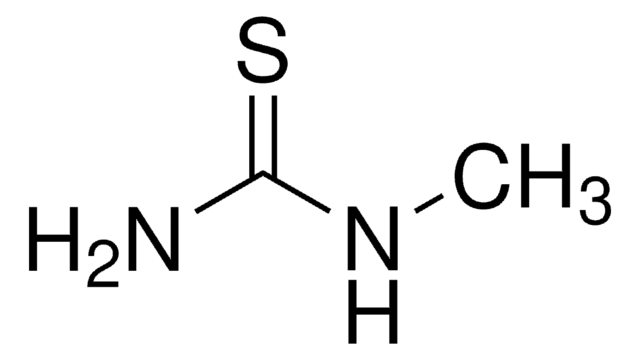
N-Methylurea
97%
Synonym(s):
1-Methylurea, N-Methylurea
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
CH3NHCONH2
CAS Number:
Molecular Weight:
74.08
Beilstein/REAXYS Number:
878189
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
97%
form
crystals
SMILES string
CNC(N)=O
InChI
1S/C2H6N2O/c1-4-2(3)5/h1H3,(H3,3,4,5)
InChI key
XGEGHDBEHXKFPX-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
M Kitano et al.
Japanese journal of cancer research : Gann, 88(9), 797-806 (1997-11-25)
For carcinogenic risk assessment of combinations of N-nitroso precursors in man, the effects of feeding methylurea (MU) or morpholine (Mor) plus sodium nitrite (NaNO2) were investigated using a multi-organ carcinogenesis model. In experiment 1, to initiate multiple organs, groups of
M Yamamoto et al.
Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association, 25(9), 663-668 (1987-09-01)
The formation of N-nitrosomethylurea (NMU) from methylurea (MU) and sodium nitrite in the guinea-pig stomach and the disappearance of NMU from the stomach were studied using a previously described method for NMU determination (Yamamoto et al. Fd Chem. Toxic. 1986
N Poklar et al.
Journal of protein chemistry, 13(3), 323-331 (1994-04-01)
The solvent denaturation of alpha-chymotrypsinogen (alpha-ctg A) in aqueous solution of urea, methyl-, N,N'-dimethyl-, ethyl-, propyl- and butylurea was studied by fluorescence measurements. Data were analyzed on the assumption of a two-state approximation to obtain the apparent equilibrium constant, Ku
Nuzhat Gull et al.
Colloids and surfaces. B, Biointerfaces, 51(1), 10-15 (2006-06-30)
The present study highlights the fact that the effect of additives (urea, monomethylurea, thiourea) on the supramolecular assemblies and proteins is strikingly similar. To investigate the effect, a viscometeric study on sphere-to-rod transition (s-->r) was undertaken in a system (3.5%
L S Povarov et al.
Bioorganicheskaia khimiia, 16(4), 559-568 (1990-04-01)
Derivatives of antitumour anthracycline antibiotics containing N-methylurea moiety in the carbohydrate ring were obtained by the interaction of methyl isocyanate with daunorubicin, doxorubicin, carminomycin and daunorubicin derivatives, substituted at C-13 or C-14 positions. N-Nitrosation of these compounds yielded modified anthracycline
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service