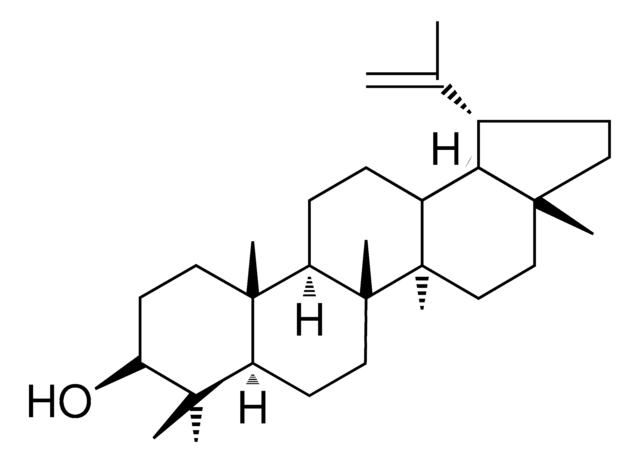
The solubility of Lupeol in DMSO is 3 mg/mL ( (7.03 mM). Moisture-absorbing DMSO reduces solubility therefore it is important to use fresh DMSO.
50 micromoles = 0.00005 moles
1 mole of Lupeol contains 426.72g, therefore 0.00005 moles contain (0.00005 moles/1mole) * 426.72g/mol) = 21.336mg
Thus, dissolving 21.336mg of Lupeol in 1 Liter of DMSO will yield a 50 micromolar solution of Lupeol.
L5632
Lupeol
≥94%
Synonym(s):
β-Viscol, 20(29)-Lupen-3β-ol, 3β-Hydroxy-20(29)-lupene, Clerodol, Fagarasterol, Lupenol, Monogynol B
Select a Size
Select a Size
About This Item
Recommended Products
Quality Level
assay
≥94% (TLC)
≥94%
storage temp.
2-8°C
SMILES string
[H]C12CCC3C4(C)CCC(O)C(C)(C)[C@]4([H])CCC3(C)[C@]1(C)CCC5(C)CC[C@H](C(C)=C)[C@]25[H]
InChI
1S/C30H50O/c1-19(2)20-11-14-27(5)17-18-29(7)21(25(20)27)9-10-23-28(6)15-13-24(31)26(3,4)22(28)12-16-30(23,29)8/h20-25,31H,1,9-18H2,2-8H3/t20-,21+,22-,23+,24-,25+,27+,28-,29+,30+/m0/s1
InChI key
MQYXUWHLBZFQQO-QGTGJCAVSA-N
Looking for similar products? Visit Product Comparison Guide
General description
signalword
Warning
hcodes
Hazard Classifications
Acute Tox. 4 Oral
Storage Class
11 - Combustible Solids
wgk_germany
WGK 1
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
-
what is the solubility of lupeol in DMSO? How can I make 50 micromolar solutions of lupeol?
1 answer-
Helpful?
-
Active Filters
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service