All Photos(2)
About This Item
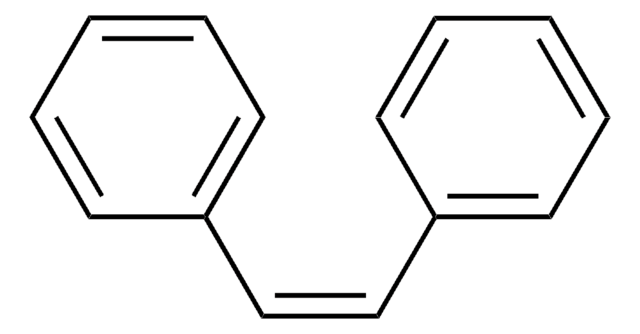
Linear Formula:
C6H5C≡CC6H5
CAS Number:
Molecular Weight:
178.23
Beilstein/REAXYS Number:
606478
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
98%
form
crystals
bp
170 °C/19 mmHg (lit.)
mp
59-61 °C (lit.)
density
0.99 g/mL at 25 °C (lit.)
SMILES string
c1ccc(cc1)C#Cc2ccccc2
InChI
1S/C14H10/c1-3-7-13(8-4-1)11-12-14-9-5-2-6-10-14/h1-10H
InChI key
JRXXLCKWQFKACW-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Wenjing Hong et al.
Journal of the American Chemical Society, 134(4), 2292-2304 (2011-12-20)
Employing a scanning tunneling microscopy based beak junction technique and mechanically controlled break junction experiments, we investigated tolane (diphenylacetylene)-type single molecular junctions having four different anchoring groups (SH, pyridyl (PY), NH(2), and CN) at a solid/liquid interface. The combination of
Athanasia C Tzika et al.
Genome biology and evolution, 7(6), 1827-1841 (2015-07-03)
Despite the availability of deep-sequencing techniques, genomic and transcriptomic data remain unevenly distributed across phylogenetic groups. For example, reptiles are poorly represented in sequence databases, hindering functional evolutionary and developmental studies in these lineages substantially more diverse than mammals. In
Jim C Spain et al.
Applied and environmental microbiology, 69(7), 4037-4042 (2003-07-04)
Several strategies for using enzymes to catalyze reactions leading to the synthesis of relatively simple substituted picolinic acids have been described. The goal of the work described here was to synthesize a more complex molecule, 6-phenylacetylene picolinic acid [6-(2-phenylethynyl)pyridine-2-carboxylic acid]
Hiroaki Imoto et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 24(35), 8797-8803 (2018-05-03)
2,3,4,5-Tetraaryl-1-phenylarsoles were synthesized by utilizing safely generated diiodophenylarsine and zirconacyclopentadienes. The obtained peraryl arsoles showed aggregation-induced emission (AIE), where intense emission was observed in the solid states (quantum yields up to 0.61), whereas the corresponding solutions were very weakly emissive.
Neola F McKinley et al.
The Journal of organic chemistry, 71(25), 9552-9555 (2006-12-02)
Carbolithiation of diphenylacetylene can be exploited to generate (E)-1-lithio-1,2-diphenylalkyl-1-enes which can be reacted in situ with triisopropylborate to stereoselectively provide (E)-1,2-diphenyl-1-alkylene boronic acids. These tetrasubstituted vinylboronic acids served as versatile intermediates for the generation of tetrasubstituted olefins with retention of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service



![4-[(4-Fluorophenyl)ethynyl]phenol AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/188/684/0b16c024-0d26-4b43-a607-60b40446e593/640/0b16c024-0d26-4b43-a607-60b40446e593.png)