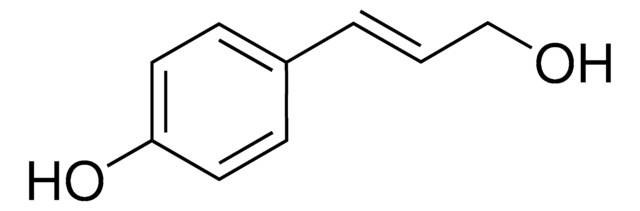
C81101
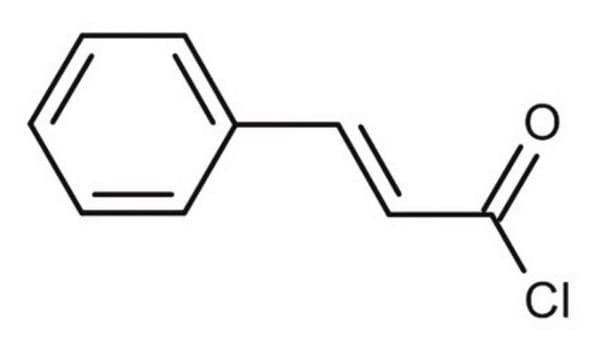
Cinnamoyl chloride

≥95.0%
Synonym(s):
trans-3-Phenylacryloyl chloride
Sign Into View Organizational & Contract Pricing
All Photos(4)
About This Item
Linear Formula:
C6H5CH=CHCOCl
CAS Number:
Molecular Weight:
166.60
Beilstein/REAXYS Number:
606265
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
≥95.0%
form
solid
bp
256-258 °C (lit.)
mp
35-37 °C (lit.)
storage temp.
2-8°C
SMILES string
ClC(=O)\C=C\c1ccccc1
InChI
1S/C9H7ClO/c10-9(11)7-6-8-4-2-1-3-5-8/h1-7H/b7-6+
InChI key
WOGITNXCNOTRLK-VOTSOKGWSA-N
Looking for similar products? Visit Product Comparison Guide
signalword
Danger
hcodes
Hazard Classifications
Skin Corr. 1B
Storage Class
8A - Combustible corrosive hazardous materials
wgk_germany
WGK 3
flash_point_f
235.4 °F - closed cup
flash_point_c
113 °C - closed cup
ppe
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Shinya Yano et al.
Carbohydrate polymers, 184, 418-426 (2018-01-22)
Biocompatibility of cinnamoyl-modified carbohydrate materials is not well-known, while they are attracting attention as a photoreactive material. In order to investigate biocompatible properties of cinnamoyl-modified carbohydrate, hydroxypropyl cellulose (HPC) was reacted with cinnamoyl chloride to yield cinnamoyl-modified HPC (HPC-C) for
Bianca Pérez et al.
Bioorganic & medicinal chemistry letters, 23(3), 610-613 (2013-01-08)
Novel 9-aminoacridine derivatives were synthesized by linking the heteroaromatic core to different cinnamic acids through an aminobutyl chain. The test compounds demonstrated mid-nanomolar in vitro activity against erythrocytic stages of the chloroquine-resistant W2 strain of the human malaria parasite Plasmodium
A Aĭtmambetov et al.
Bioorganicheskaia khimiia, 32(4), 446-447 (2006-08-17)
Interaction of 7-hydroxyisoflavonones with cinnamoyl chloride results in cinnamoyloxyisoflavonones.
María V Buchieri et al.
Bioorganic & medicinal chemistry letters, 23(3), 740-743 (2012-12-26)
A small series of C-cinnamoyl glycoside containing the phenol moiety was tested for the inhibition of the three Mycobacterium tuberculosis β-carbonic anhydrases (CAs, EC 4.2.1.1) with activities in the low micromolar range detected. The compounds were also tested for the
K A Connors et al.
Journal of pharmaceutical sciences, 72(4), 369-372 (1983-04-01)
The kinetics of reaction of trans-cinnamic anhydride or trans-cinnamoyl chloride with n-propyl alcohol, catalyzed by N-methylimidazole or 4-dimethylaminopyridine, were studied spectrophotometrically at 25 degrees in methyl ethyl ketone, ethylene dichloride, methylene chloride, and toluene. The acid chloride reacted in all
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service