79421
Phosphazene base P4-t-Bu solution
~0.8 M in hexane
Synonym(s):
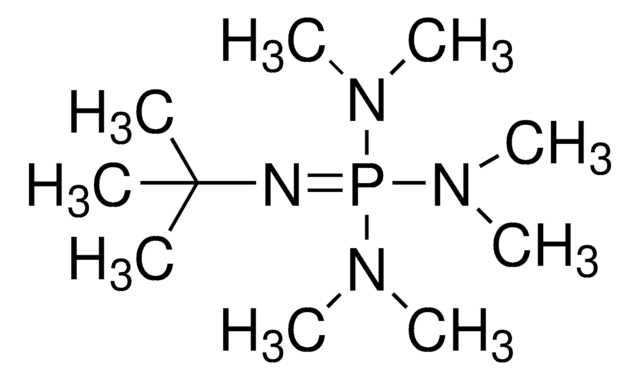
1-tert-Butyl-4,4,4-tris(dimethylamino)-2,2-bis[tris(dimethylamino)-phosphoranylidenamino]-2λ5,4λ5-catenadi(phosphazene)
About This Item
Recommended Products
form
liquid
concentration
~0.8 M in hexane
density
0.850-0.875 g/mL at 20 °C
SMILES string
CN(C)P(=NP(=NC(C)(C)C)(N=P(N(C)C)(N(C)C)N(C)C)N=P(N(C)C)(N(C)C)N(C)C)(N(C)C)N(C)C
InChI
1S/C22H63N13P4/c1-22(2,3)23-36(24-37(27(4)5,28(6)7)29(8)9,25-38(30(10)11,31(12)13)32(14)15)26-39(33(16)17,34(18)19)35(20)21/h1-21H3
InChI key
NSRBCQCXZAYQHF-UHFFFAOYSA-N
Caution
Other Notes
signalword
Danger
Hazard Classifications
Aquatic Chronic 3 - Asp. Tox. 1 - Eye Dam. 1 - Flam. Liq. 2 - Repr. 2 - Skin Corr. 1B - STOT RE 1 Inhalation - STOT SE 3
target_organs
Central nervous system, Nervous system
Storage Class
3 - Flammable liquids
wgk_germany
WGK 3
flash_point_f
-14.8 °F - closed cup
flash_point_c
-26 °C - closed cup
ppe
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
Phosphazene base reagents are available as monomeric (P1 and BEMP), dimeric (P2), and tetrameric (P4) bases with different side chains to control their sterical hindrance.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service




![1,8-Diazabicyclo[5.4.0]undec-7-ene 98%](/deepweb/assets/sigmaaldrich/product/structures/120/564/5b373e23-1624-489c-8efb-692de0f96ffb/640/5b373e23-1624-489c-8efb-692de0f96ffb.png)






