All Photos(2)
About This Item
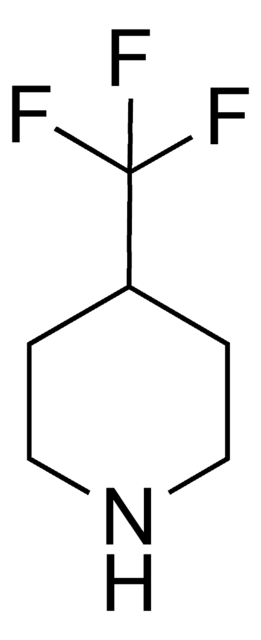
Empirical Formula (Hill Notation):
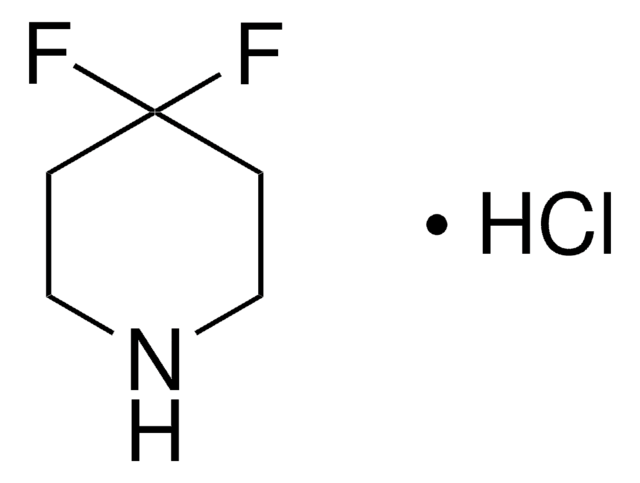
C4H7F2N · HCl
CAS Number:
Molecular Weight:
143.56
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
97%
form
solid
mp
133-136 °C
SMILES string
Cl.FC1(F)CCNC1
InChI
1S/C4H7F2N.ClH/c5-4(6)1-2-7-3-4;/h7H,1-3H2;1H
InChI key
YYVPZQADFREIFR-UHFFFAOYSA-N
Application
3,3-Difluoropyrrolidine hydrochloride can be used as a building block in the synthesis of:
It can be also used as a reactant in the preparation of cyclic and acyclic β-aminofluoroalkenes via allylic amination using the Pd catalyst.
- Triazole substituted prolyl difluoropyrrolidines as potential inhibitors of dipeptidyl peptidase-4.
- Dual leucine zipper kinase (DLK) inhibitors.
It can be also used as a reactant in the preparation of cyclic and acyclic β-aminofluoroalkenes via allylic amination using the Pd catalyst.
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Design, Synthesis, Structure-Activity Relationships, and Docking Studies of 1-(?-1, 2, 3-Triazol Substituted Prolyl)-(S)-3, 3-Difluoropyrrolidines as a Novel Series of Potent and Selective Dipeptidyl Peptidase-4 Inhibitors
Zhang L, et al.
Chemical Biology & Drug Design, 81(2), 198-207 (2013)
Activation of Allylic C-F bonds: Palladium-Catalyzed Allylic Amination of 3, 3-Difluoropropenes
Pigeon X, et al.
Angewandte Chemie (International Edition in English), 49(6), 1123-1127 (2010)
Snahel Patel et al.
Journal of medicinal chemistry, 58(1), 401-418 (2014-10-24)
Dual leucine zipper kinase (DLK, MAP3K12) was recently identified as an essential regulator of neuronal degeneration in multiple contexts. Here we describe the generation of potent and selective DLK inhibitors starting from a high-throughput screening hit. Using proposed hinge-binding interactions
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service