All Photos(2)
About This Item
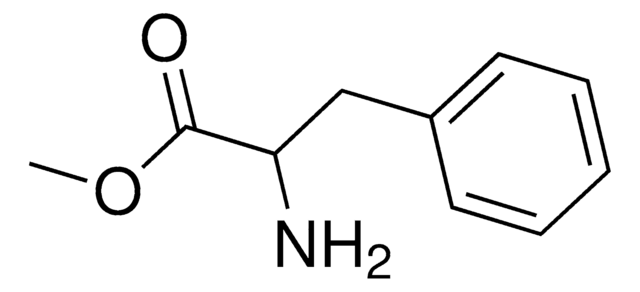
Linear Formula:
C6H5CH2CH(NH2)CO2CH3 · HCL
CAS Number:
Molecular Weight:
215.68
MDL number:
UNSPSC Code:
12352209
eCl@ss:
32160406
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
98%
reaction suitability
reaction type: solution phase peptide synthesis
mp
159-163 °C (lit.)
application(s)
peptide synthesis
storage temp.
2-8°C
SMILES string
Cl[H].COC(=O)[C@H](N)Cc1ccccc1
InChI
1S/C10H13NO2.ClH/c1-13-10(12)9(11)7-8-5-3-2-4-6-8;/h2-6,9H,7,11H2,1H3;1H/t9-;/m1./s1
InChI key
SWVMLNPDTIFDDY-SBSPUUFOSA-N
Looking for similar products? Visit Product Comparison Guide
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Andrew J Quinn et al.
Biotechnology progress, 27(6), 1554-1560 (2012-01-12)
The direct one-step synthesis of L-phenylalanine methyl ester in an organic-aqueous biphasic system using phenylalanine ammonia lyase (E.C.4.3.1.5, PAL) containing Rhodotorula glutinis yeast whole cells was reported earlier. We report here further optimization of this biotransformation using isolated PAL, when
S Reissmann et al.
Journal of medicinal chemistry, 39(4), 929-936 (1996-02-16)
For further studies on the structural and conformational requirements of positions 2,3, and 7 in the bradykinin sequence, we replaced the proline residues by the more hydrophobic and conformationally restricted N-methyl-L- and D-phenylalanine (NMF). The biological activities of the new
Y Gazitt et al.
Blood, 86(1), 381-389 (1995-07-01)
High-dose therapy with autologous marrow or peripheral blood stem cell (PBSC) rescue has been extensively applied in the treatment of multiple myeloma (MM) patients during the past 10 years resulting in improved event-free and overall survival when compared with standard
Y Watanabe et al.
Biochimica et biophysica acta, 1337(1), 40-46 (1997-01-04)
A newly isolated actinomycete belonging to Saccharothrix sp. was found to produce a unique enzyme catalyzing D-amino acid transfer. The enzyme, which was tentatively named D-amino acid transferase, was purified 2600-fold to electrophoretic homogeneity and the molecular mass was 41
L Ye et al.
Journal of molecular recognition : JMR, 11(1-6), 75-78 (1999-03-17)
We have studied the possibility of shifting a thermodynamically unfavourable enzymatic equilibrium towards product formation via the addition of a highly specific adsorbent. The commercially interesting enzymatic condensation of Z-L-aspartic acid with L-phenylalanine methyl ester to the sweetener aspartame was
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service