405191
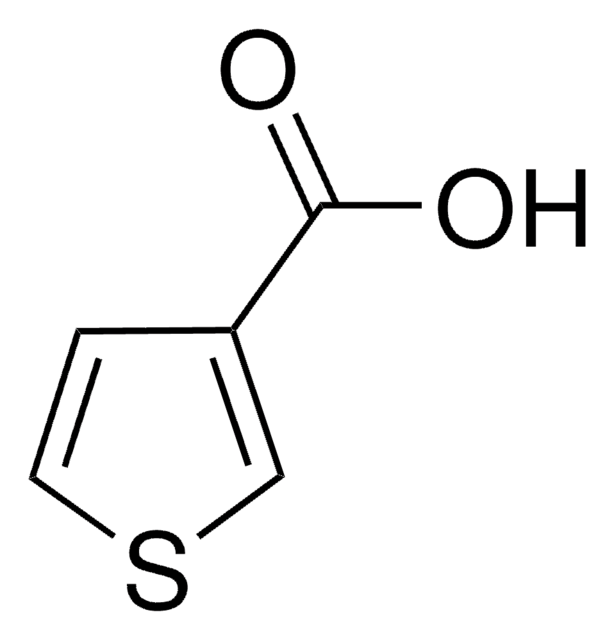
2,5-Thiophenedicarboxylic acid
99%
Synonym(s):
2,5-Dicarboxythiophene, Thiophene-2,5-dicarboxylic acid
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C6H4O4S
CAS Number:
Molecular Weight:
172.16
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
99%
mp
>300 °C (lit.)
functional group
carboxylic acid
SMILES string
OC(=O)c1ccc(s1)C(O)=O
InChI
1S/C6H4O4S/c7-5(8)3-1-2-4(11-3)6(9)10/h1-2H,(H,7,8)(H,9,10)
InChI key
YCGAZNXXGKTASZ-UHFFFAOYSA-N
General description
2,5-Thiophenedicarboxylic acid (2,5-TDCA, H2TDC) is an important building block in the preparation of fluorescent brightening agent, fungicides and anti-cancer drug. It has been generated by chlorination of adipic acid using thionyl chloride. Due to its diverse coordination modes, it is an excellent choice as an aromatic linker to generate coordination networks. The enthalpies of combustion and sublimation of 2,5-thiophenedicarboxylic acid have been evaluated by rotary-bomb combustion calorimetry.
Application
2,5-Thiophenedicarboxylic acid may be used as a thiophene based linker in the preparation of magnesium coordination networks. It may also be used as a precursor in the synthesis of 2,5-bis-benzoxazoyl-thiophene (EBF).
It may be used in the synthesis of the following:
It may be used in the synthesis of the following:
- new cobalt(II) thiophenedicarboxylate coordination polymer
- novel mesogenic thiophene based supramolecular liquid crystals
- two novel coordination polymers with the formula {[Ln2(2,5-tdc)3(dmso)2]H2O}n (Ln = Tb(III) and Dy(III)), (2,5-tdc2 = 2,5-thiophenedicarboxylate and dmso = dimethylsulfoxide)
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Aude Demessence et al.
Inorganic chemistry, 46(9), 3423-3425 (2007-03-29)
A new carboxylato-bridged CoII network of formula {Co((kappa1-kappa1)-(kappa1-mu2)-mu4-TDC)(mu2-H2O)0.5(H2O)}n (H2TDC=2,5-thiophenedicarboxylic acid) has been hydrothermally synthesized and characterized by single-crystal X-ray diffraction, thermogravimetric analysis, and IR and UV-visible spectroscopies. The title compound is made of chains of CoII dimers interconnected by TDC2-
Synthesis, characterization, and luminescence properties of magnesium coordination networks using a thiophene-based linker.
Calderone PJ, et al.
Inorgorganica Chimica Acta, 378(1), 109-114 (2011)
Devthade Vidyasagar et al.
Scientific reports, 9(1), 7186-7186 (2019-05-12)
An n-p type homostructural metal-free graphitic carbon nitride (g-C3N4) semiconductor is designed and developed for pollutant abatement and energy storage application. The successful grafting of vibrio-like morphology-based g-C3N4 by 2, 5-Thiophenedicarboxylic acid (TDA) molecule and the development of amide-type linkage
Preliminary communication Properties of nonlinear supramolecular liquid crystals containing thiophenedicarboxylic acids.
Lin H-C, et al.
Liq. Cryst., 25(2), 277-283 (1998)
Solid-liquid equilibrium and thermodynamic of 2, 5-thiophenedicarboxylic acid in different organic solvents.
Zhi W, et al.
Fluid Phase Equilibria, 375, 110-114 (2014)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service