262102
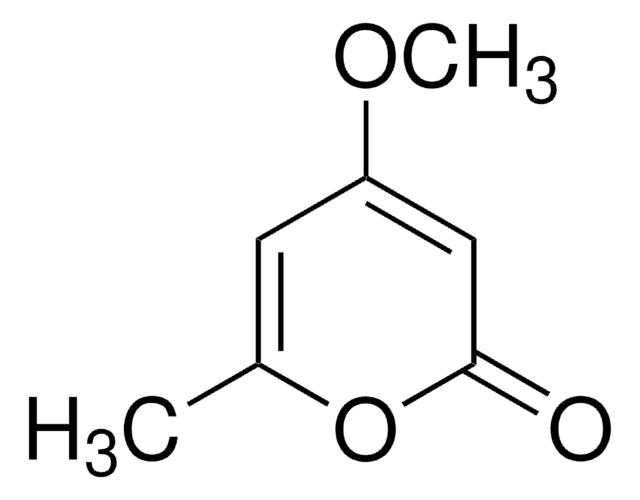
5,6-Dihydro-2H-pyran-2-one
technical grade, 90%
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C5H6O2
CAS Number:
Molecular Weight:
98.10
Beilstein/REAXYS Number:
1610
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
technical grade
Quality Level
assay
90%
form
liquid
refractive index
n20/D 1.483 (lit.)
bp
103 °C/10 mmHg (lit.)
density
1.139 g/mL at 25 °C (lit.)
storage temp.
2-8°C
SMILES string
O=C1OCCC=C1
InChI
1S/C5H6O2/c6-5-3-1-2-4-7-5/h1,3H,2,4H2
InChI key
QBDAFARLDLCWAT-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Enantioselective conjugate addition of Grignard reagents to 5,6-dihydro-2H-pyran-2-one catalyzed by a chiral phosphine-copper iodide catalyst has been reported.
Application
5,6-Dihydro-2H-pyran-2-one has been used in the preparation of:
- (1aR,5aR,5bS,6S,7S)-6,7-di-tert-butoxy-5-oxo-pyrrolidino[1,2-b]isoxazolidino[4,5-c]tetrahydropyran
- 4-(phenyl)tetrahydro-2H-pyran-2-one
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Rhodium-catalyzed asymmetric 1, 4-addition of arylboron reagents to α,β-unsaturated esters.
Takaya Y, et al.
Tetrahedron Asymmetry, 10(20), 4047-4056 (1999)
Conjugate addition of diethylzinc to a, ?-unsaturated lactones catalyzed by copper-phosphite complexes.
Yan M, et al.
Chemical Communications (Cambridge, England), 2, 115-116 (2000)
D Socha et al.
Carbohydrate research, 336(4), 315-318 (2001-12-01)
(1aR,5aR,5bS,6S,7S)-6,7-Di-tert-butoxy-5-oxo-pyrrolidino[1,2-b]isoxazolidino[4,5-c]tetrahydropyran (8) prepared by (1,3)-dipolar cycloaddition of the cyclic nitrone 6 derived from tartaric acid to 5,6-dihydro-2H-pyran-2-one (7) was transformed into indolizidine 11 via a sequence of reactions involving methanolysis of the lactone ring, intramolecular alkylation of the nitrogen atom
Fabrizio Carta et al.
Bioorganic & medicinal chemistry letters, 22(1), 267-270 (2011-12-06)
The inhibition of the zinc enzyme carbonic anhydrase (CA, EC 4.2.1.1) with (thio)coumarins has been recently reported (Maresca et al., J. Am. Chem. Soc. 2009, 131, 3057). Here we demonstrate that a series of γ- and δ-(thio)lactones also act as
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service