209511
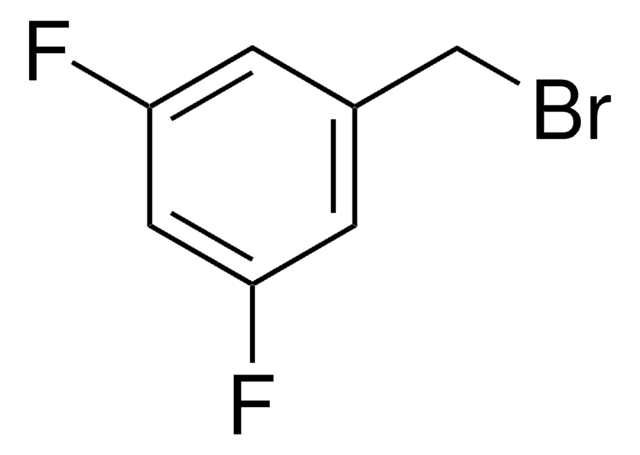
2-Fluorobenzyl bromide
98%
Synonym(s):
α-Bromo-2-fluorotoluene
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
FC6H4CH2Br
CAS Number:
Molecular Weight:
189.02
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
98%
form
liquid
refractive index
n20/D 1.552 (lit.)
bp
84-85 °C/15 mmHg (lit.)
density
1.567 g/mL at 25 °C (lit.)
functional group
bromo
fluoro
SMILES string
Fc1ccccc1CBr
InChI
1S/C7H6BrF/c8-5-6-3-1-2-4-7(6)9/h1-4H,5H2
InChI key
FFWQLZFIMNTUCZ-UHFFFAOYSA-N
Application
2-Fluorobenzyl bromide was used:
- in the synthesis of 2-pyrrolo[2,3-d]pyrimidines
- as alkylating agent during the synthesis of 8-alkylated imidazolo[1,2-a]pyrimid-5-ones
- in the synthesis of prasugrel
signalword
Danger
hcodes
Hazard Classifications
Skin Corr. 1B - STOT SE 3
target_organs
Respiratory system
Storage Class
8A - Combustible corrosive hazardous materials
wgk_germany
WGK 3
flash_point_f
181.4 °F - closed cup
flash_point_c
83 °C - closed cup
ppe
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Yun-Fei Zhu et al.
Bioorganic & medicinal chemistry letters, 12(3), 399-402 (2002-01-30)
Initial SAR studies on 1-aminomethyl-2-aryl-3-cyano-pyrrolo[1,2-a]pyrimid-7-one-6-carboxylates as human GnRH receptor antagonists were discussed. 2-(2-Methylaminoethyl)pyridine was discovered to be a key feature for generating active compounds. The best compound from the series had 25 nM (K(i)) binding affinity to human GnRH receptor.
J L Kelley et al.
Journal of medicinal chemistry, 38(19), 3884-3888 (1995-09-15)
Analogues of 9-(2-fluorobenzyl)-6-(methylamino)-9H-purine (1) containing isosteric replacements of the imidazole ring atoms were synthesized and tested for anticonvulsant activity. The pyrrolo[2,3-d]-, pyrazolo[3,4-d]-, and triazolo[4,5-d]pyrimidines were less active than 1 against maximal electroshock-induced seizures (MES) in rats when given po. The
Synthesis of prasugrel.
SUN Z-G, et al
Chinese Journal of Pharmaceuticals / Chung-kuo i yao kung yeh tsa chih, 4, 006-006 (2009)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service