138827
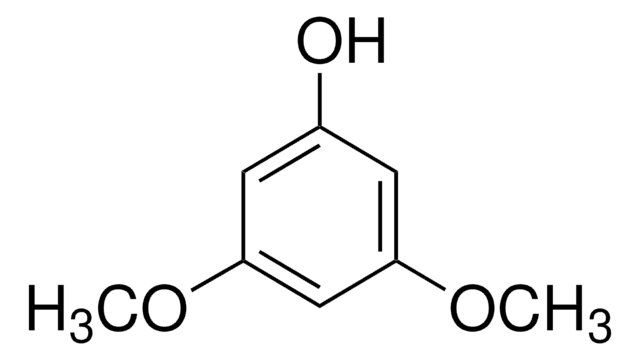
1,3,5-Trimethoxybenzene
ReagentPlus®, ≥99%
Synonym(s):
Phloroglucinol trimethyl ether
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C6H3(OCH3)3
CAS Number:
Molecular Weight:
168.19
Beilstein/REAXYS Number:
1307993
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
product line
ReagentPlus®
assay
≥99%
form
solid
bp
255 °C (lit.)
mp
50-53 °C (lit.)
SMILES string
COc1cc(OC)cc(OC)c1
InChI
1S/C9H12O3/c1-10-7-4-8(11-2)6-9(5-7)12-3/h4-6H,1-3H3
InChI key
LKUDPHPHKOZXCD-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
1,3,5-Trimethoxybenzene effectively cleaves p-methoxybenzyl protecting group on various alcohols and acids. It is the major scent compound present in Chinese rose species.
Application
1,3,5-Trimethoxybenzene was used to study the photodeoxygenation of 1,2-benzodiphenylene sulfoxide. It was employed as secondary standard in quantitative proton NMR spectroscopy of pharmaceuticals.
Legal Information
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
signalword
Warning
hcodes
Hazard Classifications
Acute Tox. 4 Oral
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
[Effects of liver disease and age on metabolism of trimethoxybenzene (author's transl)].
P Allain et al.
Therapie, 35(5), 591-595 (1980-09-01)
A N Mayeno et al.
The Journal of biological chemistry, 264(10), 5660-5668 (1989-04-05)
Human eosinophils preferentially utilize bromide to generate a brominating agent, even at physiological halide concentrations, where chloride (140 mM) is over 1000-fold greater than bromide (20-100 microM). Under the same conditions, neutrophils use chloride to generate a chlorinating agent. The
M Saeed Arayne et al.
Pakistan journal of pharmaceutical sciences, 18(4), 7-12 (2005-12-29)
A simple, sensitive, reliable, and rapid high-performance liquid chromatographic method for the simultaneous determination of phloroglucinol and trimethyl phloroglucinol has been developed. Acetonitrile-water (1:1 v/v) was used as mobile phase, with flow rate 2 ml/minutes. pH was adjusted to 3
Nicolas Kern et al.
The Journal of organic chemistry, 77(20), 9227-9235 (2012-09-26)
The p-methoxybenzyl protecting group (PMB) on various alcohols and an acid was efficiently and selectively cleaved by the action of a catalytic amount of silver(I) hexafluoroantimonate combined with 0.5 equiv of 1,3,5-trimethoxybenzene in dichloromethane at 40 °C.
O Chassany et al.
Alimentary pharmacology & therapeutics, 25(9), 1115-1123 (2007-04-19)
Abdominal pain is the predominant symptom in irritable bowel syndrome patients. Phloroglucinol and its methylated derivative are antispasmodic agents acting on smooth muscle. To evaluate the efficacy of phloroglucinol/trimethylphloroglucinol on pain intensity during an acute exacerbation of pain of irritable
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service