C2409
Creatinase from microorganisms
lyophilized powder, ≥4 units/mg solid
Synonym(s):
Creatine Amidinohydrolase
Sign Into View Organizational & Contract Pricing
All Photos(7)
About This Item
CAS Number:
EC Number:
MDL number:
UNSPSC Code:
12352204
NACRES:
NA.54
Recommended Products
biological source
bacterial (Actinobacillus spp.)
form
lyophilized powder
specific activity
≥4 units/mg solid
mol wt
~100 kDa
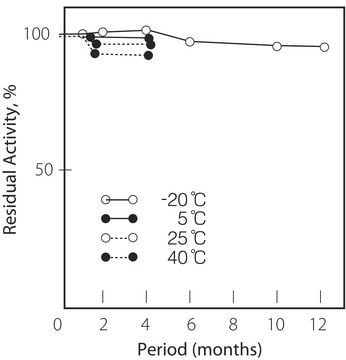
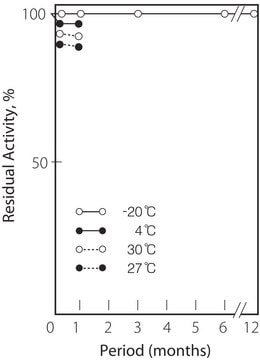
storage temp.
−20°C
General description
Creatinase is a homodimer that catalyzes hydrolysis of creatine. It consists of two monomer subunits and two defined domains; N and C terminal domains. The C-terminal fold has both the α helices and anti-parallel β sheet within two structurally similar domains.In between these two domains, a sulfhydryl group acts as active site, and the activity is metal-independent.
Application
Creatinase mixed with sarcosine oxidase may be used to determine the level of creatine in different pH, temperature, enzyme ratio, and buffer concentration. It may also be used to determine the plasma creatinine level by using a centrifugal analyser.
Biochem/physiol Actions
Creatinase accelerates the conversion reaction of creatine and water molecule to sarcosine and urea. It always acts in homodimer state and is induced by choline chloride.
Physical properties
Isoelectric point: 4.6 ± 0.1
Michaelis constant: 1.9 x 10‾2M (Creatine)
Structure: 2 subunits per mole of enzyme
Inhibitors: Cu++, Hg++, Ag+
Optimum pH: 8.0
Optimum temp: 40°C
pH Stability: pH 5.5 − 9.0 (25°C, 16hr)
Thermal stability: Below 50°C (pH 7.5, 30 min)
Michaelis constant: 1.9 x 10‾2M (Creatine)
Structure: 2 subunits per mole of enzyme
Inhibitors: Cu++, Hg++, Ag+
Optimum pH: 8.0
Optimum temp: 40°C
pH Stability: pH 5.5 − 9.0 (25°C, 16hr)
Thermal stability: Below 50°C (pH 7.5, 30 min)
Unit Definition
One unit will hydrolyze 1.0 μmole of creatine to urea and sarcosine per min at pH 7.5 at 37 °C.
Physical form
Lyophilized powder containing sugars and EDTA as stabilizers
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Resp. Sens. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
J F Bazan et al.
Proceedings of the National Academy of Sciences of the United States of America, 91(7), 2473-2477 (1994-03-29)
Amino acid sequence comparison suggests that the structure of Escherichia coli methionine aminopeptidase (EC 3.4.11.18) and the C-terminal domain of Pseudomonas putida creatinase (EC 3.5.3.3) are related. A detailed comparison of the three-dimensional folds of the two enzymes confirms this
Balasundaram Padmanabhan et al.
Acta crystallographica. Section D, Biological crystallography, 58(Pt 8), 1322-1328 (2002-07-24)
The crystal structure of Actinobacillus creatine amidinohydrolase has been solved by molecular replacement. The amino-acid sequence has been derived from the crystal structure. Crystals belong to space group I222, with unit-cell parameters a = 111.26 (3), b = 113.62 (4)
H Crocker et al.
Journal of clinical pathology, 41(5), 576-581 (1988-05-01)
An enzymatic kit method for the determination of plasma creatinine was optimised for use with a centrifugal analyser and its performance characteristics and practicability compared with an end point and a kinetic Jaffé-based method. The enzymatic method exhibited several advantages
M-L Lee
Pediatric cardiology, 26(6), 792-796 (2005-08-06)
I report on a 3-month-old infant with pulmonary atresia-intact ventricular septum and ventriculocoronary communication (VCC) who underwent percutaneous radiofrequency valvulotomy and valvuloplasty (RFVV). The patient's cardiac troponin-I, creatine kinase (CK), and myocardial fraction of (CK-MB) were elevated before RFVV and
T Yoshimoto et al.
Journal of biochemistry, 79(6), 1381-1383 (1976-06-01)
A method was developed for purification and crystallization of creatinase [creatine amidinohydrolase, EC 3.5.3.3] from Pseudomonas putida var. naraensis C-83. The purified preparation appeared homogeneous on disc electrophoresis and ultracentrifugation and had a molecular weight of 94,000. It was most
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service