B3131
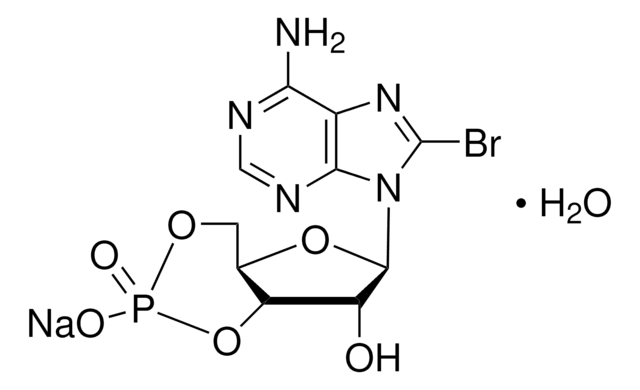
8-Bromoadenosine 5′-monophosphate
≥98%
Synonym(s):
8-Br-AMP
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C10H13BrN5O7P
CAS Number:
Molecular Weight:
426.12
MDL number:
UNSPSC Code:
41106305
PubChem Substance ID:
NACRES:
NA.51
Recommended Products
biological source
synthetic (organic)
Assay
≥98%
form
powder
solubility
water: 100 mg/mL, clear, colorless
storage temp.
−20°C
SMILES string
Nc1ncnc2n(C3OC(COP(O)(O)=O)C(O)C3O)c(Br)nc12
InChI
1S/C10H13BrN5O7P/c11-10-15-4-7(12)13-2-14-8(4)16(10)9-6(18)5(17)3(23-9)1-22-24(19,20)21/h2-3,5-6,9,17-18H,1H2,(H2,12,13,14)(H2,19,20,21)
InChI key
DNPIJKNXFSPNNY-UHFFFAOYSA-N
Related Categories
Application
8-Bromoadenosine 5′′-monophosphate (8-Br-AMP) is an analogue of 5′-AMP useful for receptor mapping studies; as a starting structure for 8-modified 5′-AMP derivatives and for synthesis of poly-8-bromoriboadenylic acid.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
J O Folayan et al.
Journal of biochemistry, 96(4), 1297-1301 (1984-10-01)
Poly-8-bromoriboadenylic acid was synthesized by the bromination of adenosine-5'-monophosphate to yield 8-bromoadenosine-5'-monophosphate which on conversion to the 5'-diphosphate form was polymerized by polynucleotide phosphorylase (PNPase). The polymer formed a 1:1 hybrid with polyribouridylic acid and the hybrid was found to
Vikram J Tallapragada et al.
The Journal of pharmacology and experimental therapeutics, 356(2), 424-433 (2015-11-19)
The ventrolateral medulla contains presympathetic and vagal preganglionic neurons that control vasomotor and cardiac vagal tone, respectively. G protein-coupled receptors influence the activity of these neurons. Gα s activates adenylyl cyclases, which drive cyclic adenosine monophosphate (cAMP)-dependent targets: protein kinase
Sophia E Airhart et al.
PloS one, 12(12), e0186459-e0186459 (2017-12-07)
The co-primary objectives of this study were to determine the human pharmacokinetics (PK) of oral NR and the effect of NR on whole blood nicotinamide adenine dinucleotide (NAD+) levels. Though mitochondrial dysfunction plays a critical role in the development and
Charlotte H E Weimar et al.
PloS one, 7(7), e41424-e41424 (2012-08-01)
The aetiology of recurrent miscarriage (RM) remains largely unexplained. Women with RM have a shorter time to pregnancy interval than normally fertile women, which may be due to more frequent implantation of non-viable embryos. We hypothesized that human endometrial stromal
Rongkuan Hu et al.
PloS one, 8(8), e73527-e73527 (2013-08-24)
This study is the first to demonstrate that shizukaol D, a natural compound isolated from Chloranthusjaponicus, can activate AMP- activated protein kinase (AMPK), a key sensor and regulator of intracellular energy metabolism, leading to a decrease in triglyceride and cholesterol
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service