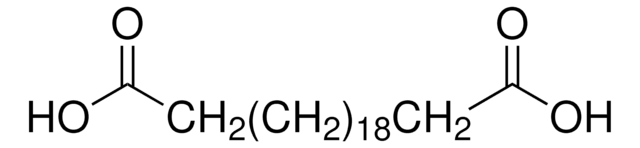60930
Suberic acid
purum, ≥98.0% (T)
Synonym(s):
Octanedioic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
HOOC(CH2)6COOH
CAS Number:
Molecular Weight:
174.19
Beilstein:
1210161
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
purum
Quality Level
Assay
≥98.0% (T)
bp
230 °C/15 mmHg (lit.)
mp
140-144 °C (lit.)
140-144 °C
SMILES string
OC(=O)CCCCCCC(O)=O
InChI
1S/C8H14O4/c9-7(10)5-3-1-2-4-6-8(11)12/h1-6H2,(H,9,10)(H,11,12)
InChI key
TYFQFVWCELRYAO-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
410.0 °F - closed cup
Flash Point(C)
210 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
S Hayakawa
Zeitschrift fur allgemeine Mikrobiologie, 22(5), 309-326 (1982-01-01)
Through the isolation and identification of a wide variety of degradation products formed from bile acids by microorganisms, a unified scheme for the complete degradation of bile acids to carbon dioxide and water has been proposed and discussed. The proposed
Monica Ilies et al.
Bioorganic & medicinal chemistry, 11(10), 2227-2239 (2003-04-26)
Novel matrix metalloproteinase (MMP)/bacterial collagenase inhibitors are reported, considering the sulfonylated amino acid hydroxamates as lead molecules. A series of compounds was prepared by reaction of arylsulfonyl isocyanates with N-(5H-dibenzo[a,d]cyclohepten-5-yl)- and N-(10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5-yl) methyl glycocolate, respectively, followed by the conversion of
Larisa Sheihet et al.
Biomacromolecules, 6(5), 2726-2731 (2005-09-13)
We describe the synthesis and characterization of a family of biocompatible ABA-triblock copolymers that comprised of hydrophilic A-blocks of poly(ethylene glycol) and hydrophobic B-blocks of oligomers of suberic acid and desaminotyrosyl-tyrosine esters. The triblock copolymers spontaneously self-assemble in aqueous solution
M Rivard et al.
Amino acids, 15(4), 389-392 (1999-01-19)
Papain-catalyzed regioselective cleavage of alpha-methyl ester in Z-DL-Asu(OMe)-OMe leads to Z-L-Asu(OMe)-OH and Z-D-Asu(OMe)-OMe. Subsequent saponifications yield Z-L-Asu-OH and Z-D-Asu-OH. The enzymatic alpha-ester hydrolysis was also achieved by subtilisin BPN' in organic solvent with low water content.
Taishi Yokoi et al.
Dalton transactions (Cambridge, England : 2003), 41(9), 2732-2737 (2012-01-18)
Octacalcium phosphates (OCPs) co-incorporated with various molar ratios of succinate and suberate ions were synthesized by wet processing. The interplanar spacings of the (100) planes (d(100)) of OCPs formed in the presence of succinic acid (Suc) or suberic acid (Sub)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service