All Photos(1)
About This Item
Linear Formula:
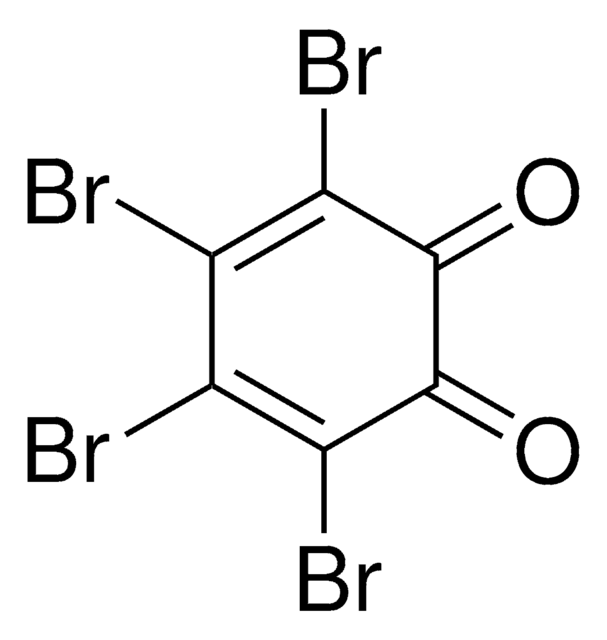
C6Cl4-1,2-(OH)2
CAS Number:
Molecular Weight:
247.89
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
technical
Quality Level
Assay
≥95.0% (HPLC)
mp
193-195 °C (lit.)
SMILES string
Oc1c(O)c(Cl)c(Cl)c(Cl)c1Cl
InChI
1S/C6H2Cl4O2/c7-1-2(8)4(10)6(12)5(11)3(1)9/h11-12H
InChI key
RRBMVWQICIXSEO-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Tetrachlorocatechol is a metabolite of pentachlorophenol. Acute toxicity of tetrachlorocatechol has been investigated in male and female mice.
Application
Tetrachlorocatechol was used to investigate the inhibition of various ureases by halogenated benzo- and naphthoquinones, potent inhibitors of pure ureases from Bacillus pasteurii and Canavalia ensiformis. It may be used to prepare the copper(II) complexes of the potentially tripodal N,N,O ligand 3,3-bis(1-methylimidazol-2-yl)propionate (L1) and its conjugate acid HL1.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Acute 1 - Eye Dam. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Acute toxicities of pentachlorophenol, pentachloroanisole, tetrachlorohydroquinone, tetrachlorocatechol, tetrachlororesorcinol, tetrachlorodimethoxybenzenes and tetrachlorobenzenediol diacetates administered to mice.
Renner G, et al.
Toxicological & Environmental Chemistry, 11(1), 37-50 (1986)
Shaogui Yang et al.
Journal of hazardous materials, 161(2-3), 1281-1287 (2008-06-17)
A novel photocatalysis material, F-Si-comodified TiO(2) (FST) powder, was synthesized by ultrasound-assisted hydrolysis. The prepared material was characterized by X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS) and UV-visible absorption spectroscopy, respectively. XRD analysis indicated that the phase of FST was
Smadar Levy et al.
Environmental toxicology and chemistry, 28(7), 1380-1389 (2009-02-14)
Pentachlorophenol (PCP) is used in industrial and domestic applications, including as a biocide and a wood preservative. Metabolism of PCP undergoes oxidative dechlorination, forming tetrachlorocatechol (TCC) and tetrachlorohydroquinone (TCHQ). Both sodium azide (NaN(3)) and TCC appear naturally in soil. None
I-H Nam et al.
Applied microbiology and biotechnology, 62(2-3), 284-290 (2003-07-29)
Pseudomonas veronii PH-05, a bacterial strain capable of transforming pentachlorophenol (PCP) to a metabolic intermediate, was isolated by selective enrichment of soil samples from a timber storage yard. Strain PH-05 was shown to be able to grow using PCP as
Hyo-Bong Hong et al.
Applied and environmental microbiology, 68(5), 2584-2588 (2002-04-27)
Aerobic biotransformation of the diaryl ethers 2,7-dichlorodibenzo-p-dioxin and 1,2,3,4-tetrachlorodibenzo-p-dioxin by the dibenzo-p-dioxin-utilizing strain Sphingomonas wittichii RW1, producing corresponding metabolites, was demonstrated for the first time. Our strain transformed 2,7-dichlorodibenzo-p-dioxin, yielding 4-chlorocatechol, and 1,2,3,4-tetrachlorodibenzo-p-dioxin, producing 3,4,5,6-tetrachlorocatechol and 2-methoxy-3,4,5,6-tetrachlorophenol; all of these
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service