All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C10H9N3
CAS Number:
Molecular Weight:
171.20
Beilstein:
127131
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
solid
bp
222 °C/50 mmHg (lit.)
mp
90-92 °C (lit.)
SMILES string
N(c1ccccn1)c2ccccn2
InChI
1S/C10H9N3/c1-3-7-11-9(5-1)13-10-6-2-4-8-12-10/h1-8H,(H,11,12,13)
InChI key
HMMPCBAWTWYFLR-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
2,2′-Dipyridylamine (bipyam) can be used:
- As a bidentate N-donor ligand in the synthesis of various metal complexes.
- To synthesize Pd-polyoxovanadates, heterogeneous catalyst for the oxidation of benzylic hydrocarbons.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Evaluation of DNA, BSA, and HSA binding propensity of copper (II) complex with N-donor ligand 2, 2′-dipyridylamine
Kumar M and Masram DT
Polyhedron, 157, 511-520 (2019)
Structural diversity of manganese (II) complexes containing 2, 2′-dipyridylamine and benzenedicarboxylates. Conformational analysis of tere-, iso-and phthalate ions: An experimental and quantum chemical approach
Radovanovic L, et al.
Inorgorganica Chimica Acta, 445, 46-56 (2016)
Venugopal Rajendiran et al.
Dalton transactions (Cambridge, England : 2003), (16)(16), 2157-2170 (2008-04-10)
A series of mixed ligand ruthenium(II) complexes [Ru(Hdpa)2(diimine)](ClO4)2, 1-5 where Hdpa is 2,2'-dipyridylamine and diimine is 1,10-phenanthroline (phen) and a modified/extended 1,10-phenanthroline such as, 5,6-dimethyl-1,10-phenanthroline (5,6-dmp), dipyrido[3,2-d:2',3'-f]quinoxaline (dpq), 5-methyldipyrido[3,2-d:2',3'-f]quinoxaline (mdpq) and dipyrido[3,2-a:2',3'-c]phenazine (dppz) have been isolated and characterized by analytical
Sanela Martić et al.
Inorganic chemistry, 47(18), 8315-8323 (2008-08-20)
The syntheses of new blue luminescent N(2)-modified guanosine derivatives with chromophores p-4,4'-biphenyl-NPh2 (1a), p-4,4'-biphenyl-N(2-py)2 (1b), and p-4,4'-biphenyl-2-(2'-pyridyl)benzimidazolyl (1c), respectively, have been achieved. These new N(2)-guanosines are moderate blue emitters with lambda(max) = 395 nm (1a), 370 nm (1b), and 403
Mohan N Patel et al.
Journal of enzyme inhibition and medicinal chemistry, 26(2), 188-197 (2010-06-30)
The copper(II) complexes of the type [Cu(SPF)(A(n))Cl]/[Cu(PFL)(A(n))Cl] (where SPF is sparfloxacin, PFL is pefloxacin and A(n) is 2,2'-dipyridylamine/pyridine-2-carboxalehyde/thiophene-2-carboxaldehyde) were synthesised and were found to have a pyramidal geometry with a square base. The superoxide dismutase (SOD) like activity of the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service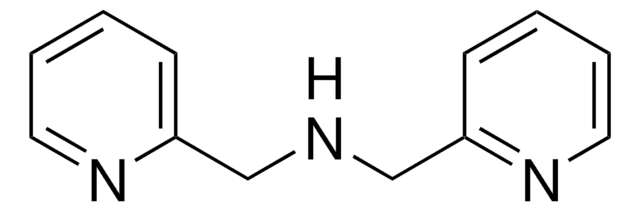












![2-[(Methylamino)methyl]pyridine 97%](/deepweb/assets/sigmaaldrich/product/structures/370/687/248fbc0c-2e59-447c-8191-2685dfb597d6/640/248fbc0c-2e59-447c-8191-2685dfb597d6.png)