746959
Ethenesulfonyl fluoride
95%
Synonym(s):
ESF, Vinyl sulfonyl fluoride
About This Item
Recommended Products
Quality Level
Assay
95%
form
liquid
reaction suitability
reaction type: click chemistry
refractive index
n20/D 1.385
density
1.328 g/mL at 25 °C
SMILES string
O=S(F)(C=C)=O
InChI
1S/C2H3FO2S/c1-2-6(3,4)5/h2H,1H2
InChI key
BYPHZHGVWNKAFC-UHFFFAOYSA-N
General description
Application
- β-Arylethenesulfonyl fluorides via palladium(II) acetate-catalyzed Heck-Matsuda reaction with arenediazonium tetrafluoroborates.
- Bisalkylsulfonyl fluoride(BSF) monomers via Michael addition reaction with amines/anilines. BSF monomers are applicable in the synthesis of polysulfonates.
- Cyclobutane-fused pyridinyl sulfonyl fluorides by photocatalytic [2 + 2] cycloaddition with pyridones or isoquinolones.
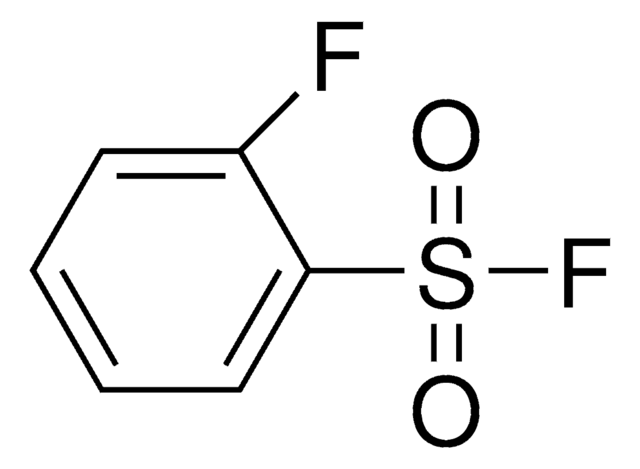
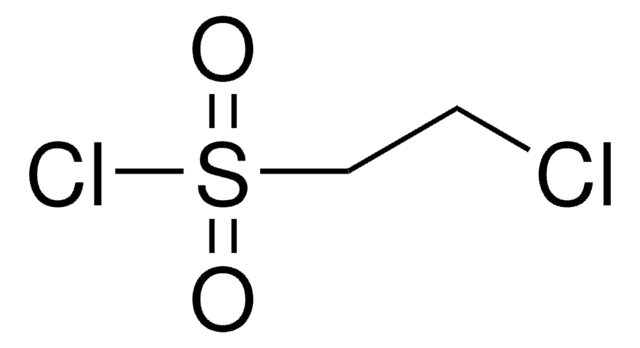
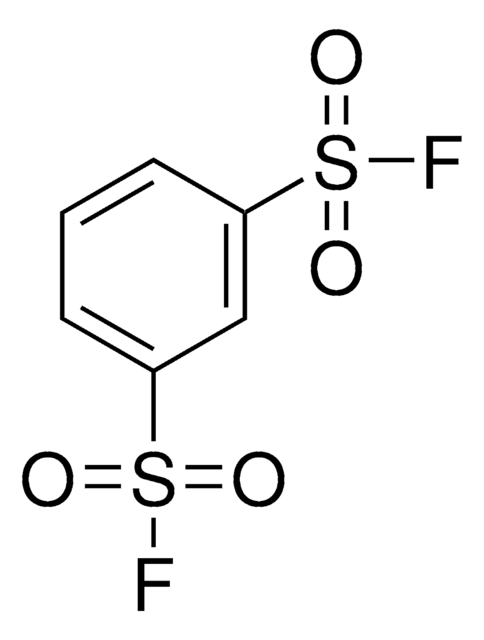
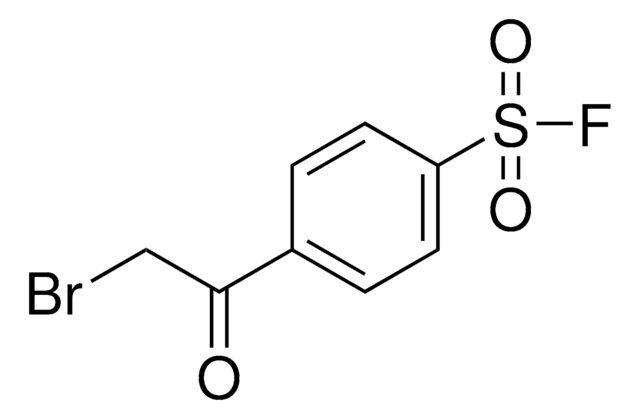
related product
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Eye Dam. 1 - Flam. Liq. 3 - Muta. 2 - Skin Corr. 1B - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
104.0 °F - closed cup
Flash Point(C)
40 °C - closed cup
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
In collaboration with K. Barry Sharpless and coworkers, we offer a variety of sulfonyl fluoride reagents that undergo a “Click II” reaction.
In collaboration with K. Barry Sharpless and coworkers, we offer a variety of sulfonyl fluoride reagents that undergo a “Click II” reaction.
In collaboration with K. Barry Sharpless and coworkers, we offer a variety of sulfonyl fluoride reagents that undergo a “Click II” reaction.
In collaboration with K. Barry Sharpless and coworkers, we offer a variety of sulfonyl fluoride reagents that undergo a “Click II” reaction.
Related Content
The Sharpless Lab pursues useful new reactivity and general methods for selectively controlling chemical reactions.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service