All Photos(1)
About This Item
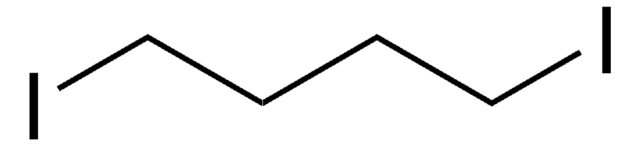
Linear Formula:
I(CF2)4I
CAS Number:
Molecular Weight:
453.84
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
refractive index
n20/D 1.429 (lit.)
bp
150 °C (lit.)
mp
−9 °C (lit.)
density
2.474 g/mL at 25 °C (lit.)
SMILES string
FC(F)(I)C(F)(F)C(F)(F)C(F)(F)I
InChI
1S/C4F8I2/c5-1(6,3(9,10)13)2(7,8)4(11,12)14
InChI key
JILAKKYYZPDQBE-UHFFFAOYSA-N
General description
Octafluoro-1,4-diiodobutane (ofib, C4F8I2) is also referred to as 1,1,2,2,3,3,4,4-octafluoro-1,4-diiodobutane. It acts as a halogen bond donor compound and its cocrystallization with methyldiphenylphosphine oxide (mdppo) has been investigated. In combination with a calixcrown compound ofib forms a ′binary host′ system, which is employed for the isolation of cesium iodide from aqueous to fluorous phase.
Application
Octafluoro-1,4-diiodobutane may be used as a guest molecule to prepare a chiral bidentate inclusion complex with pyridyl moieties of a pyridoallenoacetylenic host. It may be employed in the synthesis of three-component supramolecular complexes.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Metric engineering of supramolecular Borromean rings.
Liantonio R, et al.
Chemical Communications (Cambridge, England), 17, 1819-1821 (2006)
Dipyridinocalixcrown/diiodoperfluorocarbon binary host systems for CsI: structural studies and fluorous phase extraction of caesium.
Gattuso G, et al.
Tetrahedron, 63(23), 4951-4958 (2007)
Silvia Castro-Fernández et al.
Organic letters, 16(4), 1136-1139 (2014-02-12)
A chiral bidentate inclusion complex has been formed by halogen-bond interaction between the pyridyl moieties of a pyridoallenoacetylenic host and octafluorodiiodobutane. X-ray crystallography showed that the guest adopts a chiral conformation inside the molecular channels formed by stacking of the
Switching between halogen-and hydrogen-bonding in stoichiometric variations of a cocrystal of a phosphine oxide.
Oh SY, et al.
CrystEngComm, 14(19), 6110-6114 (2012)
Liang Lu et al.
Journal of applied toxicology : JAT, 39(7), 945-954 (2019-03-06)
Fluorinated diiodine alkanes (FDIAs), important industrial intermediates in the synthesis of various perfluorinated compounds, which are distributed widely in wildlife and humans. Recent studies showed that FDIAs had in vitro estrogenic effects. However, to date, little information is available regarding
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service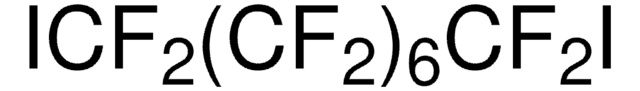


![N-[4-(3,3,4,4,5,5,6,6,7,7,8,8,9,9,10,10,10-Heptadecafluorodecyl) benzyloxycarbonyloxy]succinimide ≥97.0% (NMR)](/deepweb/assets/sigmaaldrich/product/structures/232/278/7cb408c4-7b05-4cf3-8e6d-6886bb873b9c/640/7cb408c4-7b05-4cf3-8e6d-6886bb873b9c.png)