All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C4H5NO3
CAS Number:
Molecular Weight:
115.09
MDL number:
UNSPSC Code:
12352005
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
solid
optical activity
[α]20/D −46°, c = 1 in methanol
mp
99-102 °C (lit.)
SMILES string
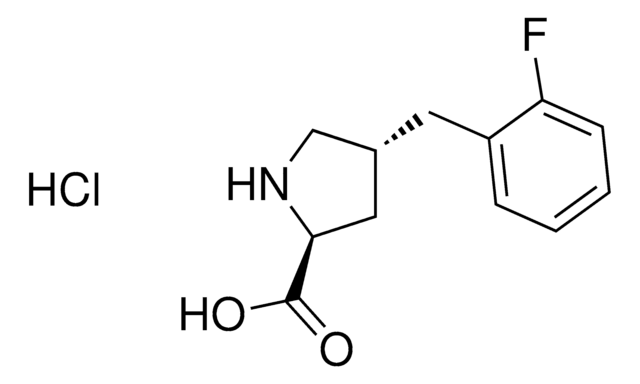
OC(=O)[C@@H]1CC(=O)N1
InChI
1S/C4H5NO3/c6-3-1-2(5-3)4(7)8/h2H,1H2,(H,5,6)(H,7,8)/t2-/m0/s1
InChI key
YSPMLLKKKHCTBN-REOHCLBHSA-N
General description
The crystals of (S)-(-)-4-oxo-2-azetidinecarboxylic acid are orthorhombic and belongs to P212121 space group.
Application
Important building block for the synthesis of NMDA receptor antagonists, 3-alkyl-L-aspartic acids, and orally active β-lactam inhibitors.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Structures of (S)-(-)-4-oxo-2-azetidinecarboxylic acid and 3-azetidinecarboxylic acid from powder synchrotron diffraction data.
Mora AJ, et al.
Acta Crystallographica Section B, Structural Science, Crystal Engineering and Materials, 62(4), 606-611 (2006)
Baldwin, J.E. et al.
Tetrahedron, 51, 11581-11581 (1995)
Hanessian, S. et al.
Synlett, 33-33 (1992)
P E Finke et al.
Journal of medicinal chemistry, 38(13), 2449-2462 (1995-06-23)
The stereospecific synthesis of several 4-[(4-carboxyphenyl)oxy]- 3,3-dialkyl-1-[[(1-phenylalkyl)-amino]carbonyl]azetidin-2-on es 3 is described in which the C-3 alkyl groups were varied from methyl to butyl as well as allyl, benzyl and methoxymethyl. The structure-activity relations for these compounds are discussed in terms
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service