SRP9000
Prolactin human
human, recombinant, expressed in HEK 293 cells
Synonym(s):
PRL
About This Item
Recommended Products
biological source
human
Quality Level
recombinant
expressed in HEK 293 cells
sterility
non-sterile
Assay
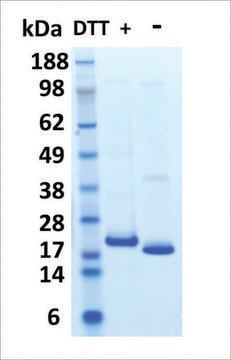
≥95% (SDS-PAGE)
form
liquid
potency
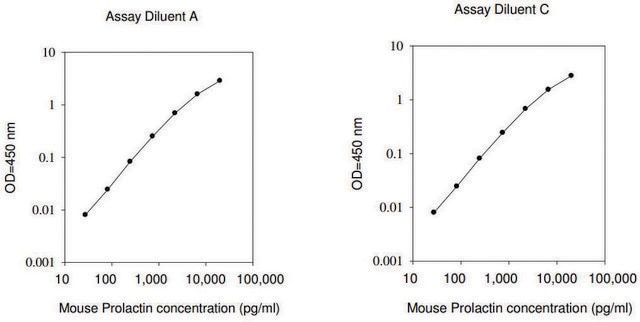
≤2 ng/mL Nb2-11 cells proliferation EC50
shelf life
2 yr
mol wt
23 kDa
technique(s)
cell culture | mammalian: suitable
impurities
≤1 EU/μg protein Endotoxin level
storage temp.
−20°C
Gene Information
human ... prl(5617)
General description
Prolactin (PRL) is a multifunctional polypeptide hormone primarily produced by the lactotrophic cells of the anterior pituitary gland in vertebrates.
Application
- in in vitro experiments to examine its effects in sleep-like concentrations on T-cell migration
- to study its effects on claudin 2 (CLDN2) expression in the Caco-2 intestinal epithelial cell model
- in microplate assays to demonstrate the specificity of the antibodies for vasoinhibin
Biochem/physiol Actions
Physical form
Preparation Note
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Repr. 1B
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service