D214752
Dipropylamine
99%
Synonym(s):
Di-n-propylamine, Dipropanamine, Dipropylamine (8CI), N,N-Di(n-propyl)amine, N,N-Dipropylamine, N-Propyl-1-propanamine, n-Dipropylamine
About This Item
Recommended Products
Assay
99%
form
liquid
refractive index
n20/D 1.4049 (lit.)
bp
105-110 °C (lit.)
density
0.738 g/mL at 25 °C (lit.)
SMILES string
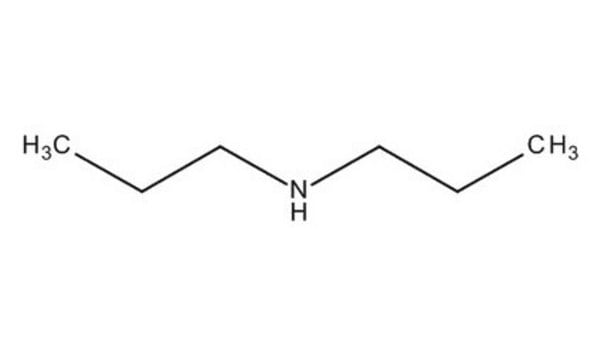
CCCNCCC
InChI
1S/C6H15N/c1-3-5-7-6-4-2/h7H,3-6H2,1-2H3
InChI key
WEHWNAOGRSTTBQ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
- Separation of hydrocarbons: Nitrogen-containing switchable solvents, including dipropylamine, are used for the separation of hydrocarbons and their derivatives, showcasing the compound′s versatility in solvent applications (Liu et al., 2020).
- Microextraction techniques: Dipropylamine-based dispersive micro-solid phase extraction combined with switchable hydrophilicity solvent is employed for enriching non-steroidal anti-inflammatory drugs from environmental water samples, demonstrating its utility in complex sample preparation (Di et al., 2020).
- Homogeneous liquid-liquid microextraction: A new pH assisted homogeneous liquid-liquid microextraction method utilizing dipropylamine as a solvent with switchable hydrophilicity was developed for the GC-MS determination of methamphetamine, highlighting its effectiveness in analytical chemistry applications (Shahvandi et al., 2018).
- Removal of contaminants: Research demonstrated the use of zeolite and powdered activated carbon in conjunction with dipropylamine for the simultaneous removal of ammonia and N-nitrosamine precursors from high ammonia water, underscoring its environmental application (Xue et al., 2018).
- Nanosized synthesis: Dipropylamine was used in a co-templating synthesis approach for the production of high-yield nanosized (Si)AlPO-41 using ethanol polarity equalization, illustrating its role in materials science (Majano et al., 2015).
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 4 Oral - Eye Dam. 1 - Flam. Liq. 2 - Skin Corr. 1A - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 1
Flash Point(F)
44.6 °F - closed cup
Flash Point(C)
7 °C - closed cup
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service