All Photos(2)
About This Item
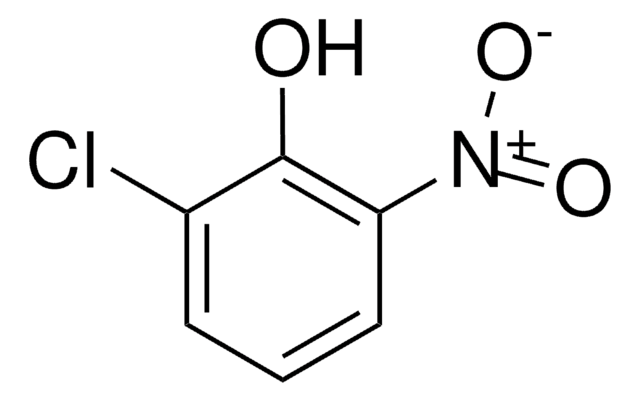
Linear Formula:
ClC6H3(NO2)OH
CAS Number:
Molecular Weight:
173.55
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
powder
mp
105-106 °C (lit.)
SMILES string
Oc1ccc(cc1Cl)[N+]([O-])=O
InChI
1S/C6H4ClNO3/c7-5-3-4(8(10)11)1-2-6(5)9/h1-3,9H
InChI key
BOFRXDMCQRTGII-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Hassan Sakhtah et al.
Applied and environmental microbiology, 85(14) (2019-05-06)
The yeast Kluyveromyces lactis has been a successful host for the production of heterologous proteins for over 30 years. Currently, the galactose-/lactose-inducible and glucose-repressible LAC4 promoter (P
Tangrong Liu et al.
Analytical and bioanalytical chemistry, 411(7), 1467-1477 (2019-02-02)
α-L-Fucosidase (AFU) is a promising therapeutic target for the treatment of inflammation, cancer, cystic fibrosis, and fucosidosis. Some of the existing analytical methods for the assessment of AFU activity are lacking in sensitivity and selectivity, since most of them are
G I Konoshenko et al.
Antibiotiki, 27(9), 687-693 (1982-01-01)
When used in concentrations of 10-15 micrograms per 10(8) cells, flavofungin and nigrofungin, carbonyl-conjugated pentaenes lowered the rate of oxygen absorption by thymocyte suspensions in the presence of glucose. Flavopentin inhibited glucose oxidation in higher concentrations. ADP and succinate did
Y A Soushko et al.
Revue de laryngologie - otologie - rhinologie, 114(1), 59-61 (1993-01-01)
In vitro experiments, Terrilytinum* increases the antimycotic activity of Clotrimazolum* and Nitrofungin*, and cancels out the inhibitory effect of Contrycal*. The combined application of Clotrimazolum and Nitrofungin with Terrilytinum and Contrycal as treatment of otomycosis of the middle ear has
V G Arzumanyan et al.
Bulletin of experimental biology and medicine, 131(4), 346-349 (2001-09-11)
Viability of 40 Candida spp. cultures was studied after long-term exposure to antifungal drugs in minimum inhibitory concentrations. The fungicidal effect decreased in the series: pimafucin-nitrofungin-diflucan-orungal-levorine-clotrimazole-exoderil. Nizoral in a concentration of 4 microg/ml was ineffective; in the rest cultures the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service