902675
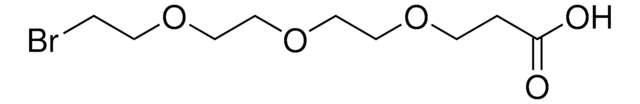
BocNH-PEG6-acid
Synonym(s):
2,2-Dimethyl-4-oxo-3,8,11,14,17,20,23-heptaoxa-5-azapentacosan-25-oic acid, Boc-NH-PEG6-CH2COOH
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C19H37NO10
CAS Number:
Molecular Weight:
439.50
MDL number:
UNSPSC Code:
12352106
NACRES:
NA.22
Recommended Products
form
liquid
reaction suitability
reagent type: linker
refractive index
n/D 1.4657
functional group
Boc
amine
carboxylic acid
storage temp.
−20°C
SMILES string
OC(COCCOCCOCCOCCOCCOCCNC(OC(C)(C)C)=O)=O
Application
This heterobifunctional, PEGylated crosslinker features a carboxylic acid at one end and Boc-protected amino group at the other, which can be deprotected with mildly acidic conditions. The hydrophillic PEG linker facilitates solubility in biological applications. BocNH-PEG6-acid can be used for bioconjugation or as a building block for synthesis of small molecules, conjugates of small molecules and/or biomolecules, or other tool compounds for chemical biology and medicinal chemistry that require ligation. Examples of applications include its synthetic incorporation into antibody-drug conjugates or proteolysis-targeting chimeras (PROTAC® molecules) for targeted protein degradation.
Other Notes
Legal Information
PROTAC is a registered trademark of Arvinas Operations, Inc., and is used under license
related product
Product No.
Description
Pricing
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Vincent Rerat et al.
Journal of medicinal chemistry, 52(22), 7029-7043 (2009-10-29)
RGD peptides are used in biomaterials science for surface modifications with a view to elicit selective cellular responses. Our objective is to replace peptides by small peptidomimetics acting similarly. We designed novel molecules targeting alpha(v)beta(3) integrin and featuring spacer-arms (for
Vincent Rerat et al.
Bioorganic & medicinal chemistry letters, 20(6), 1861-1865 (2010-02-23)
Ultrasmall particles of iron oxide (USPIOs) coated with 3,3'-bis(phosphonate)propionic acid were covalently coupled to a home-made Arg-Gly-Asp (RGD) peptidomimetic molecule via a short oligoethylene-glycol (OEG) spacer. The conjugation rate was measured by X-ray photoelectron spectroscopy (XPS). The particle size and
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service