753092
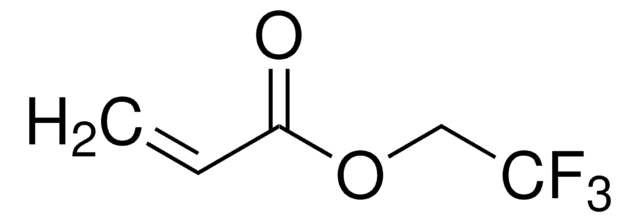
Pentafluorophenyl acrylate
contains <200 ppm monomethyl ether hydroquinone as inhibitor, 98%
Synonym(s):
2-Propenoic acid pentafluorophenyl ester
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C9H3F5O2
CAS Number:
Molecular Weight:
238.11
MDL number:
UNSPSC Code:
12162002
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Quality Level
Assay
98%
form
liquid
contains
<200 ppm monomethyl ether hydroquinone as inhibitor
refractive index
n/D 1.434
density
1.446
storage temp.
2-8°C
SMILES string
Fc1c(F)c(F)c(OC(=O)C=C)c(F)c1F
InChI
1S/C9H3F5O2/c1-2-3(15)16-9-7(13)5(11)4(10)6(12)8(9)14/h2H,1H2
InChI key
RFOWDPMCXHVGET-UHFFFAOYSA-N
Related Categories
Application
Acrylate monomer with an activated ester for coupling (esterification/amidation) reactions
Pentafluorophenyl acrylate may be used in the preparation of highly porous polymers (poly HIPs). Pentafluorophenyl acrylate (PFPA) can undergo reversible addition fragmentation transfer (RAFT) polymerization with oligoethylene glycol acrylate (OEGA) or diethylene glycol acrylate (DEGA).
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
154.4 °F - closed cup
Flash Point(C)
68 °C - closed cup
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Reactive thiol-ene emulsion-templated porous polymers incorporating pentafluorophenyl acrylate
Kircher L, et al.
Polymer, 54(7), 1755-1761 (2013)
Factors influencing the synthesis and the post-modification of PEGylated pentafluorophenyl acrylate containing copolymers
Beija M, et al.
European Polymer Journal, 49, 3060?3071-3060?3071 (2013)
Yuwaporn Pinyakit et al.
Journal of materials chemistry. B, 8(3), 454-464 (2019-12-14)
Recently, pH-responsive polymeric micelles have gained significant attention as effective carriers for anti-cancer drug delivery. Herein, pH-responsive polymeric micelles were constructed by a simple post-polymerization modification of a single homopolymer, poly(pentafluorophenyl acrylate) (PPFPA). The PPFPA was first subjected to modification
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service




![4-Cyano-4-[(dodecylsulfanylthiocarbonyl)sulfanyl]pentanoic acid 97% (HPLC)](/deepweb/assets/sigmaaldrich/product/structures/204/925/30ae6ca0-5b0b-4963-a061-7e5e3d1a85af/640/30ae6ca0-5b0b-4963-a061-7e5e3d1a85af.png)

