All Photos(1)
About This Item
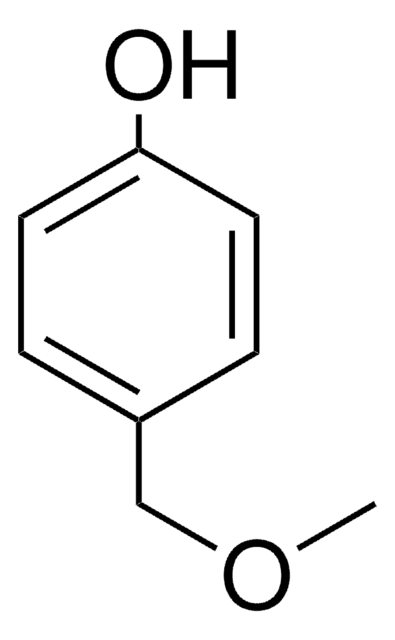
Linear Formula:
CH3O(CH2)2C6H4OH
CAS Number:
Molecular Weight:
152.19
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
mp
42-45 °C (lit.)
SMILES string
COCCc1ccc(O)cc1
InChI
1S/C9H12O2/c1-11-7-6-8-2-4-9(10)5-3-8/h2-5,10H,6-7H2,1H3
InChI key
FAYGEALAEQKPDI-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
4-(2-Methoxyethyl)phenol can be prepared by reacting methyl vinyl ether and 4-bromonitrobenzene.
Application
4-(2-Methoxyethyl)phenol may be used in the preparation of methyl analog of metoprolol (MAM).
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
230.0 °F - closed cup
Flash Point(C)
110 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
A New Route to 4-(2-Methoxyethyl) Phenol Via Palladium-Catalysed Arylation of Methyl Vinyl Ether.
Hallberg A, et al.
Synthetic Communications, 15(13), 1131-1136 (1985)
Alfred Svan et al.
Journal of mass spectrometry : JMS, 51(3), 207-218 (2016-03-10)
Identification of degradation products from trace organic compounds, which may retain the biological activity of the parent compound, is an important step in understanding the long-term effects of these compounds on the environment. Constructed wetlands have been successfully utilized to
M Allaoua et al.
Journal of applied microbiology, 125(4), 1162-1174 (2018-05-18)
In vitro and in vivo studies were conducted to test a new carvacrol-based product designed to delay the carvacrol release so that it could reach the caeca of broiler chickens in order to control Campylobacter jejuni. Antimicrobial activity of carvacrol, a
Evidence that serine 304 is not a key ligand-binding residue in the active site of cytochrome P450 2D6.
Ellis SW, et al.
The Biochemical Journal, 345(3), 565-571 (2000)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service