All Photos(2)
About This Item
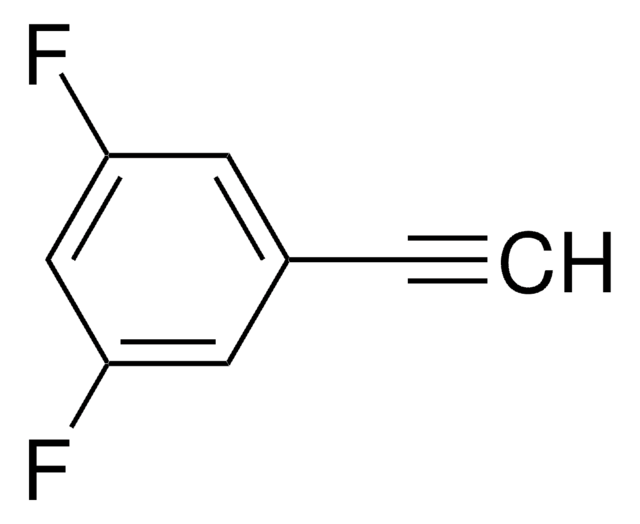
Linear Formula:
FC6H4C≡CH
CAS Number:
Molecular Weight:
120.12
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
refractive index
n20/D 1.5170 (lit.)
bp
138 °C (lit.)
density
1.039 g/mL at 25 °C (lit.)
SMILES string
Fc1cccc(c1)C#C
InChI
1S/C8H5F/c1-2-7-4-3-5-8(9)6-7/h1,3-6H
InChI key
PTRUTZFCVFUTMW-UHFFFAOYSA-N
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 3 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
90.0 °F - closed cup
Flash Point(C)
32.2 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
(NHC) Copper (I)-Catalyzed [3+ 2] Cycloaddition of Azides and Mono-or Disubstituted Alkynes.
Diez-Gonzalez S, et al.
Chemistry (Weinheim An Der Bergstrasse, Germany), 12(29), 7558-7564 (2006)
Jason Manka et al.
Probe Reports from the NIH Molecular Libraries Program, 2011 Dec 16 (Updated 2013 Mar 7) (2013-06-14)
Allosteric modulators for G-protein-coupled receptors (GPCRs) provide numerous advantages over orthosteric ligands, including greater sub-type selectivity, reduced receptor desensitization, saturability of effect, and potential for enhanced therapeutic index. Positive allosteric modulators (PAMs) of the group I metabotropic glutamate receptor mGlu
Chunfa Xu et al.
Angewandte Chemie (International ed. in English), 53(35), 9316-9320 (2014-07-22)
A new, electrophilic trifluoromethylthiolating reagent, N-trifluoromethylthiosaccharin, was developed and can be synthesized in two steps from saccharin within 30 minutes. N-trifluoromethylthiosaccharin is a powerful trifluoromethylthiolating reagent and allows the trifluoromethylthiolation of a variety of nucleophiles such as alcohols, amines, thiols, electron-rich
Ya Zhou et al.
Probe Reports from the NIH Molecular Libraries Program, 2011 Oct 31 (Updated 2013 Mar 7) (2013-06-14)
A series of acetylenic biaryl mGlu5 positive allosteric modulators (PAMs) have been optimized as pure potentiators in low receptor expressing mGlu5 cell lines. ML254 was identified and shown to competitively interact with the MPEP allosteric binding site. Preliminary data from
Gold film-catalysed benzannulation by microwave-assisted, continuous flow organic synthesis (MACOS).
Gjergji Shore et al.
Beilstein journal of organic chemistry, 5, 35-35 (2009-09-25)
Methodology has been developed for laying down a thin gold-on-silver film on the inner surface of glass capillaries for the purpose of catalysing benzannulation reactions. The cycloaddition precursors are flowed through these capillaries while the metal film is being heated
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service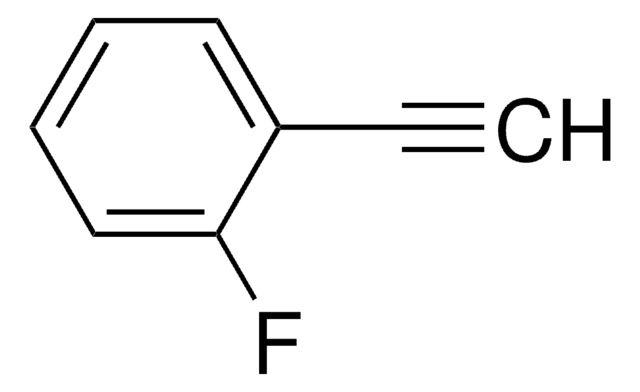






![1-[(Trimethylsilyl)ethynyl]-4-(trifluoromethyl)benzene 97%](/deepweb/assets/sigmaaldrich/product/structures/738/524/1dbb534f-6783-4963-ac51-9bf29c099aaa/640/1dbb534f-6783-4963-ac51-9bf29c099aaa.png)