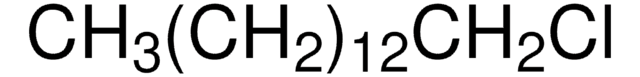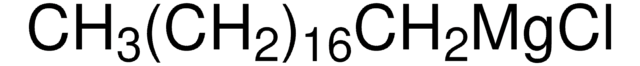238368
1-Chlorooctadecane
96%
Synonym(s):
Octadecyl chloride, Stearyl chloride
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3(CH2)16CH2Cl
CAS Number:
Molecular Weight:
288.94
Beilstein:
1703350
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
96%
form
liquid
refractive index
n20/D 1.451 (lit.)
bp
157-158 °C/1.5 mmHg (lit.)
density
0.849 g/mL at 25 °C (lit.)
functional group
alkyl halide
chloro
SMILES string
CCCCCCCCCCCCCCCCCCCl
InChI
1S/C18H37Cl/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19/h2-18H2,1H3
InChI key
VUQPJRPDRDVQMN-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Kinetic and thermodynamic analysis of catalytic hydrodechlorination of 1-chlorooctadecane in supercritical carbon dioxide using 5% Pd supported on γ-Al2O3 has been reported. Incorporation of 1-chlorooctadecane into a host monolayer of stearic acid has been reported.
Application
1-Chlorooctadecane has been used as internal standard for the determination of endocrine disrupters in water samples by stir bar sorptive extraction method.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Chronic 4 - Skin Irrit. 2
Storage Class Code
10 - Combustible liquids
WGK
WGK 1
Flash Point(F)
230.0 °F - closed cup
Flash Point(C)
110 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Catalytic hydrodechlorination of 1-chlorooctadecane, 9, 10-dichlorostearic acid, and 12, 14-dichlorodehydroabietic acid in supercritical carbon dioxide.
Aikawa B, et al.
Applied Catalysis. B, Environmental, 43(4), 371-387 (2003)
A Peñalver et al.
Journal of chromatography. A, 1007(1-2), 1-9 (2003-08-20)
Stir bar sorptive extraction (SBSE) combined with gas chromatography (GC) with mass spectrometric detection (MS) has been applied to determine a group of suspected endocrine disrupters in water samples. One centimeter stir bars coated with PDMS were used to extract
The production of stable monolayers from nonamphiphilic 1-chlorooctadecane.
Peters A and Rogers K.
Journal of Colloid and Interface Science, 157(2), 511-512 (1993)
Abid Hussain et al.
Molecules (Basel, Switzerland), 25(8) (2020-04-25)
Date palm dust mites are important pests severely infesting valuable nutritious fruits (dates) of date palm. In search of an alternative to acaricides, joint action of Metarhizium anisopliae EBCL 02049 spores and 1-Chlorooctadecane was evaluated as a potential candidate for
Elisabeth Aubert et al.
Lipids, 39(1), 75-79 (2004-04-02)
The lipids of the gram-negative marine bacterium Marinobacter hydrocarbonoclasticus, cultivated in synthetic seawater supplemented with 1-chlorooctadecane as sole source of carbon, were isolated, purified, and their structures determined. Three pools of lipids were isolated according to the sequential procedure used:
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service