All Photos(1)
About This Item
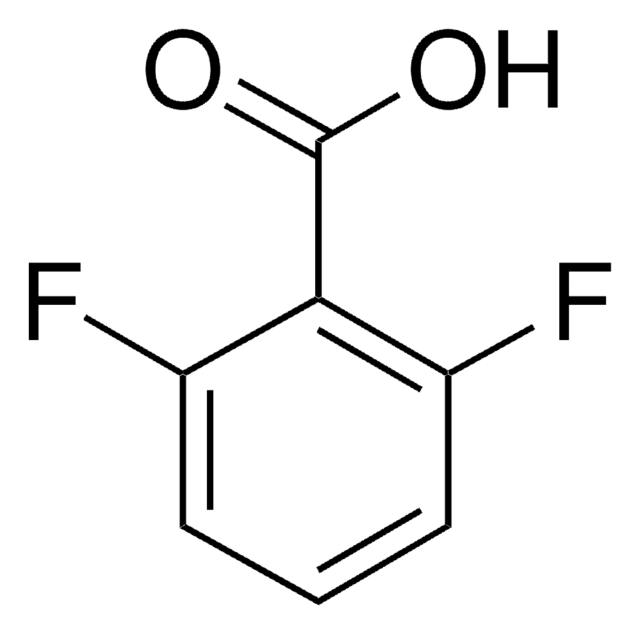
Linear Formula:
F2C6H3CN
CAS Number:
Molecular Weight:
139.10
Beilstein:
2045292
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
solid
refractive index
n20/D 1.4875 (lit.)
bp
197-198 °C
mp
25-28 °C (lit.)
density
1.246 g/mL at 25 °C (lit.)
functional group
fluoro
nitrile
SMILES string
Fc1cccc(F)c1C#N
InChI
1S/C7H3F2N/c8-6-2-1-3-7(9)5(6)4-10/h1-3H
InChI key
BNBRIFIJRKJGEI-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
2,6-Difluorobenzonitrile was used in the synthesis of:
- poly(cyanoaryl ethers) via silyl-method
- 2-dimethylamino-6-fluorobenzamide
- phenolphthalein-modified polyarylene ether nitrile copolymers
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
194.0 °F - closed cup
Flash Point(C)
90 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis and laboratory evaluation of 1-(2,6-disubstituted benzoyl)-3-phenylureas, a new class of insecticides. II. Influence of the acyl moiety on insecticidal activity.
K Wellinga et al.
Journal of agricultural and food chemistry, 21(6), 993-998 (1973-11-01)
New polymer syntheses, 15 Syntheses of aromatic polyethers from difluorobenzonitriles and silylated diphenols.
Kricheldorf HR, et al.
Makromol. Chem., Rapid Commun., 8(11), 529-534 (1987)
Xiaocan Liu et al.
Polymers, 11(9) (2019-08-31)
Porous materials with high specific surface area possess a broad application prospect in the treatment of wastewater. In this work, sulfonated poly(arylene ether nitrile) (SPEN) functionalized with a carboxylic acid group was successfully synthesized, which was subsequently transformed into SPEN
Synthesis and properties of phenolphthalein-based polyarylene ether nitrile copolymers.
Li C, et al.
Materials Letters, 60(1), 137-141 (2006)
Chenchen Liu et al.
Polymers, 11(7) (2019-07-07)
Poly(arylene ether nitrile)s with sulfonic and carboxylic groups (SCPEN) were synthesized to investigate their electrical properties. This new series of copolymers were prepared by copolymerization of phenolphthalein, potassium hydroquinonesulfonate, and 2,6-difluorobenzonitrile, in different mole ratios. Their thermal, mechanical and dielectric
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service