156353
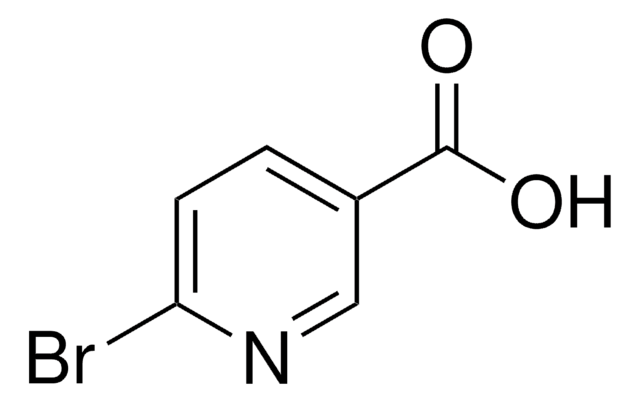
6-Chloropyridine-3-carboxylic acid
99%
Synonym(s):
6-Chloronicotinic acid
Sign Into View Organizational & Contract Pricing
All Photos(4)
About This Item
Empirical Formula (Hill Notation):
C6H4ClNO2
CAS Number:
Molecular Weight:
157.55
Beilstein:
115993
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
mp
190 °C (dec.) (lit.)
solubility
deionized water: soluble
functional group
carboxylic acid
chloro
SMILES string
OC(=O)c1ccc(Cl)nc1
InChI
1S/C6H4ClNO2/c7-5-2-1-4(3-8-5)6(9)10/h1-3H,(H,9,10)
InChI key
UAWMVMPAYRWUFX-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
6-Chloropyridine-3-carboxylic acid (6-chloronicotinic acid/6-CNA) has been used to study its photolytic and photocatalytic degradation. 6-CNA is a degradation product of neonicotinoid insecticides imidacloprid and acetamiprid and is known to appear in different environmental matrices. The product has been used as a media component during the isolation of 6-CNA degrading bacterial strain from imidacloprid-exposed soil samples.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Mahrous M Kandil et al.
Journal of agricultural and food chemistry, 63(19), 4721-4727 (2015-05-02)
Thus far, only a small number and types of bacteria with limited ability in degrading imidacloprid have been reported. Also, genes regulating imidacloprid (IMDA) degradation have yet to be discovered. To study this in more detail, an enrichment technique was
Gretty Ettiene et al.
Electrophoresis, 33(19-20), 2969-2977 (2012-09-22)
A sensitive and reliable method based on MEKC has been developed and validated for trace determination of neonicotinoid insecticides (thiamethoxam, acetamiprid, and imidacloprid) and the metabolite 6-chloronicotinic acid in water and soil matrices. Optimum separation of the neonicotinoid insecticides was
F J Uroz et al.
The Analyst, 126(8), 1355-1358 (2001-09-06)
A new analytical method for determining 6-chloronicotinic acid (6-ClNA) in human urine is proposed. 6-ClNA is the main metabolite in warm-blooded animals after exposure to the insecticide imidachloprid. 6-ClNA was extracted from human urine using solid phase extraction (SPE) with
Fabienne Hoffmann-Emery et al.
The Journal of organic chemistry, 71(5), 2000-2008 (2006-02-25)
A new efficient synthesis of two novel classes of NK1 receptor antagonists, among them befetupitant and netupitant, starting from 6-chloronicotinic acid is described. The introduction of the o-tolyl substituent at C4 of the pyridine ring was achieved by a one-pot
Romina Zabar et al.
Chemosphere, 85(5), 861-868 (2011-08-02)
This work describes for the first time the photolytic and photocatalytic degradation of 6-chloronicotinic acid (6CNA) in double deionised water, which is a degradation product of neonicotinoid insecticides imidacloprid and acetamiprid, and it is known to appear in different environmental
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service