P49805
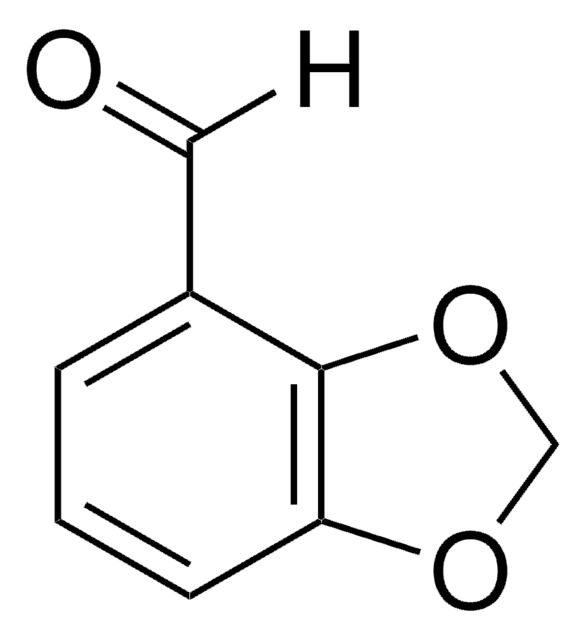
Piperonylic acid
99%
Synonym(s):
1,3-Benzodioxole-5-carboxylic acid, 3,4-(Methylenedioxy)benzoic acid
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C8H6O4
CAS Number:
Molecular Weight:
166.13
Beilstein:
150206
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
form
powder
mp
229-231 °C (lit.)
SMILES string
OC(=O)c1ccc2OCOc2c1
InChI
1S/C8H6O4/c9-8(10)5-1-2-6-7(3-5)12-4-11-6/h1-3H,4H2,(H,9,10)
InChI key
VDVJGIYXDVPQLP-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Guillaume A Schoch et al.
Plant physiology, 130(2), 1022-1031 (2002-10-12)
The cinnamate (CA) 4-hydroxylase (C4H) is a cytochrome P450 that catalyzes the second step of the main phenylpropanoid pathway, leading to the synthesis of lignin, pigments, and many defense molecules. Salicylic acid (SA) is an essential trigger of plant disease
Yue Zhu et al.
Journal of the American Chemical Society, 128(13), 4267-4276 (2006-03-30)
Two-photon excitation (2PE) of "caged" biomolecules represents a powerful method to investigate the temporal and spatial relevance of physiological function in real time and on living tissue, because the excitation volume can be restricted to 1 fL. Additionally, low-energy IR
R Ranga Rao et al.
Bioorganic & medicinal chemistry, 17(14), 5170-5175 (2009-06-12)
A bioassay-guided fractionation and chemical examination of antihyperglycemic root extract of Derris indica resulted in isolation and characterization of two new furanoflavanoids (1, 2) along with thirteen known compounds (3-15). Their structures were determined on the basis of extensive spectroscopic
J Chong et al.
Plant physiology, 125(1), 318-328 (2001-01-12)
Salicylic acid (SA) is a key endogenous component of local and systemic disease resistance in plants. In this study, we investigated the role of benzoic acid (BA) as precursor of SA biosynthesis in tobacco (Nicotiana tabacum cv Samsun NN) plants
Eyal Shimoni et al.
Journal of biotechnology, 105(1-2), 61-70 (2003-09-27)
Propenylbenzenes are often used as starting materials in the chemical synthesis of aroma compounds and fine chemicals. In the present study, we demonstrate the ability of an Arthrobacter sp. to transform various structures of propenylbenzenes derived from essential oils to
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service