655856
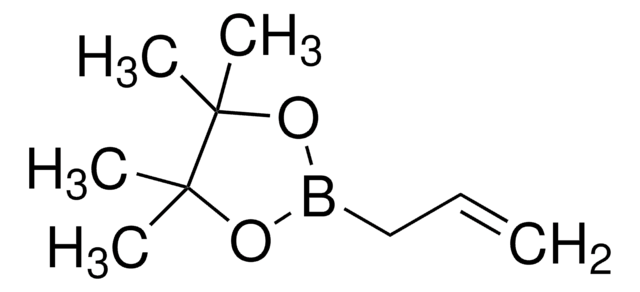
4,4,5,5-Tetramethyl-1,3,2-dioxaborolane
97%
Synonym(s):
HBpin, Pinacolborane
About This Item
Recommended Products
Quality Level
Assay
97%
refractive index
n20/D 1.396 (lit.)
bp
42-43 °C/50 mmHg (lit.)
density
0.882 g/mL at 25 °C (lit.)
storage temp.
2-8°C
SMILES string
CC1(C)OBOC1(C)C
InChI
1S/C6H13BO2/c1-5(2)6(3,4)9-7-8-5/h7H,1-4H3
InChI key
UCFSYHMCKWNKAH-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
- Borylation at the benzylic C-H bond of alkylbenzenes in the presence of a palladium catalyst to form pinacol benzyl boronate.
- Hydroboration of alkyl or aryl alkynes and alkenes in the presence of transition metal catalysts.
- Coupling with aryl iodides in the presence of a copper catalyst to form aryl boronates.
- Asymmetric hydroboration of 1,3-enynes to form chiral allenyl boronates.
related product
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Liq. 2 - Water-react 2
Storage Class Code
4.3 - Hazardous materials which set free flammable gases upon contact with water
WGK
WGK 3
Flash Point(F)
41.0 °F - closed cup
Flash Point(C)
5 °C - closed cup
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Arylboronic acids and esters, vital tools in chemical transformations, find extensive use, particularly in the Suzuki-Miyaura cross-coupling reaction.
Arylboronic acids and esters, vital tools in chemical transformations, find extensive use, particularly in the Suzuki-Miyaura cross-coupling reaction.
Arylboronic acids and esters, vital tools in chemical transformations, find extensive use, particularly in the Suzuki-Miyaura cross-coupling reaction.
Arylboronic acids and esters, vital tools in chemical transformations, find extensive use, particularly in the Suzuki-Miyaura cross-coupling reaction.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service





![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)




![Bis[(pinacolato)boryl]methane](/deepweb/assets/sigmaaldrich/product/structures/286/283/dcb13110-c536-4223-99e6-0dd505906b64/640/dcb13110-c536-4223-99e6-0dd505906b64.png)