All Photos(1)
About This Item
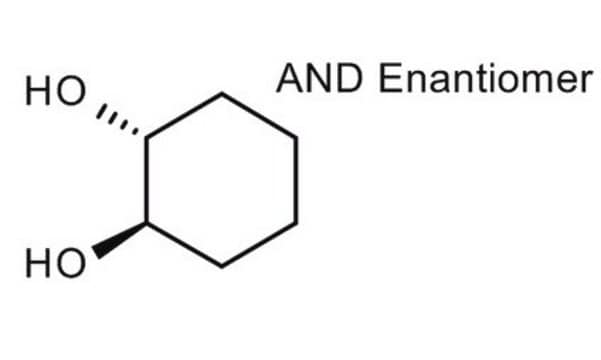
Linear Formula:
C6H10(OH)2
CAS Number:
Molecular Weight:
116.16
Beilstein:
1340578
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
mp
97-101 °C (lit.)
SMILES string
O[C@@H]1CCCC[C@@H]1O
InChI
1S/C6H12O2/c7-5-3-1-2-4-6(5)8/h5-8H,1-4H2/t5-,6+
InChI key
PFURGBBHAOXLIO-OLQVQODUSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Core-shell-like silica nickel species nanoparticle catalyzed dehydrogenation of 1,2-cyclohexanediol to catechol is reported. Crystal structure of a Cr(V) complex with cis-1,2-cyclohexanediol is reported. Enzymatic oxidation of cis-1,2-cyclohexanediol by Gluconobacter oxydans (ATCC 621) is reported.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Oxidation of trans-and cis-1, 2-cyclohexanediol by Gluconobacter oxydans.
Adlercreutz P.
Applied Microbiology and Biotechnology, 30(3), 257-263 (1989)
Ruben Bartholomäus et al.
Inorganic chemistry, 52(8), 4282-4292 (2013-03-28)
The stabilization of Cr(V) by biological 1,2-diolato ligands, including carbohydrates, glycoproteins, and sialic acid derivatives, is likely to play a crucial role in the genotoxicity of Cr(VI) and has also been implicated in the antidiabetic effect of Cr(III). Previously, such
Bao-Hui Chen et al.
Dalton transactions (Cambridge, England : 2003), 44(3), 1023-1038 (2014-11-20)
A simple and convenient approach denoted as gel-deposition-precipitation (G-D-P) for the preparation of core-shell-like silica@nickel species nanoparticles was studied systematically. Core-shell-like silica@nickel species nanoparticles consisted of a Si-rich core and a Ni-rich shell. The G-D-P process included two steps: one
Yoshihito Shiota et al.
Inorganic chemistry, 50(13), 6200-6209 (2011-06-04)
The catalytic conversion of 1,2-cyclohexanediol to adipic anhydride by Ru(IV)O(tpa) (tpa ═ tris(2-pyridylmethyl)amine) is discussed using density functional theory calculations. The whole reaction is divided into three steps: (1) formation of α-hydroxy cyclohexanone by dehydrogenation of cyclohexanediol, (2) formation of
Roosmarijn E Vandenbroucke et al.
Nucleic acids research, 35(12), e86-e86 (2007-06-23)
One of the major obstacles in non-viral gene transfer is the nuclear membrane. Attempts to improve the transport of DNA to the nucleus through the use of nuclear localization signals or importin-beta have achieved limited success. It has been proposed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service