144428
2,6-Lutidine-α2,3-diol
99%
Synonym(s):
3-Hydroxy-6-methyl-2-pyridinemethanol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C7H9NO2
CAS Number:
Molecular Weight:
139.15
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
powder
mp
157-159 °C (lit.)
SMILES string
Cc1ccc(O)c(CO)n1
InChI
1S/C7H9NO2/c1-5-2-3-7(10)6(4-9)8-5/h2-3,9-10H,4H2,1H3
InChI key
PAGTXDLKXRBHFL-UHFFFAOYSA-N
General description
2,6-Lutidine-α2,3-diol (3-Hydroxy-6-methyl-2-pyridinemethanol) on condensation with chlorides of carbamidophosphoric acids yields N-Substituted N′-[6-methyl-2-oxido-1,3,2-dioxaphosphinino(5,4,-b)pyridine-2-yl]urea. It forms organotin (IV) complexes on reaction with dimethyl-, diethyl- and dibutyltin (IV) oxide.
Application
2,6-Lutidine-α2,3-diol (3-Hydroxy-6-methyl-2-pyridinemethanol) was used in the synthesis of:
- (3-hydroxy-6-methylpyridin-2-yl)methyl pivaloate
- triflate
- (3-(allyloxy)-6-methylpyridin-2-yl)methanol
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Synthesis, structure and cytotoxicity of diorganotin (IV) complexes of 2, 6-lutidine-a2, 3-diol (Lu): The crystal structures of Lu and [SnMe2 (H2O)(Lu-2H)].
Casas JS, et al.
Journal of Organometallic Chemistry, 692(16), 3547-3554 (2007)
James R Vyvyan et al.
Synthesis, 2010(21), 3637-3644 (2011-04-26)
Palladium-catalyzed Suzuki-type couplings of 3-pyridyl triflates with alkenyl pinacol boronates proceed in good to excellent yield. Optimized conditions use Pd(PPh(3))(4) (10 mol %) as catalyst with K(3)PO(4) (3 equiv) as base in dioxane.
Synthesis and antimicrobial activity of N-substituted N'-[6-methyl-2-oxido-1, 3, 2-dioxaphosphinino (5, 4-b) pyridine-2-yl] ureas.
Reddy PC, et al.
Heteroatom Chem., 14(6), 509-512 (2003)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service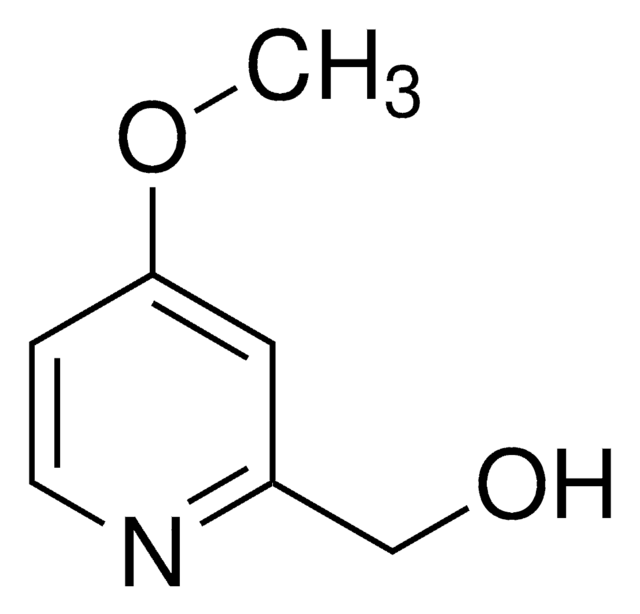






![1,4-Diazabicyclo[2.2.2]octane ReagentPlus®, ≥99%](/deepweb/assets/sigmaaldrich/product/structures/366/129/a6ff4175-974d-4fac-9038-b35e508ef252/640/a6ff4175-974d-4fac-9038-b35e508ef252.png)

