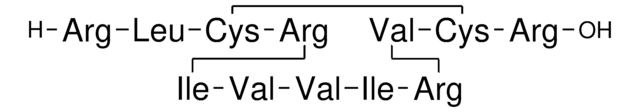About This Item
推薦產品
生物源
Streptomyces cinnamoneus
品質等級
化驗
≥95% (HPLC)
形狀
solid
溶解度
DMSO: 10 mg/mL
acetonitrile: water (1:1): 5 mg/mL (requires heating)
抗生素活性譜
fungi
作用方式
cell membrane | interferes
儲存溫度
2-8°C
InChI
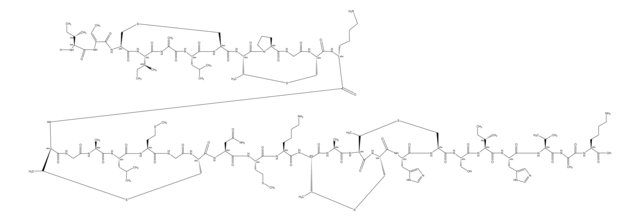
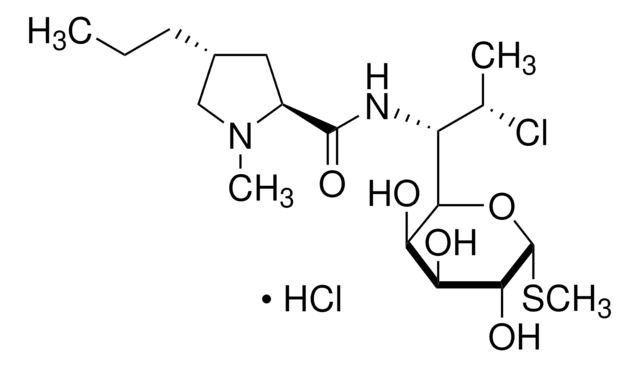
1S/C89H125N25O25S3/c1-43(2)66-84(133)109-59-41-140-40-58-79(128)108-60-42-142-45(4)68(86(135)105-55(75(124)110-66)34-48-22-12-7-13-23-48)111-76(125)54(33-47-20-10-6-11-21-47)104-82(131)61-26-17-31-114(61)65(118)38-98-72(121)53(32-46-18-8-5-9-19-46)103-78(127)57(106-80(60)129)36-95-29-15-14-24-52(87(136)137)102-85(134)67(112-77(126)56(35-63(92)116)99-64(117)37-97-83(132)69(113-81(59)130)70(119)88(138)139)44(3)141-39-49(90)71(120)100-50(25-16-30-96-89(93)94)73(122)101-51(74(123)107-58)27-28-62(91)115/h5-13,18-23,43-45,49-61,66-70,95,119H,14-17,24-42,90H2,1-4H3,(H2,91,115)(H2,92,116)(H,97,132)(H,98,121)(H,99,117)(H,100,120)(H,101,122)(H,102,134)(H,103,127)(H,104,131)(H,105,135)(H,106,129)(H,107,123)(H,108,128)(H,109,133)(H,110,124)(H,111,125)(H,112,126)(H,113,130)(H,136,137)(H,138,139)(H4,93,94,96)/t44-,45?,49+,50+,51+,52+,53+,54+,55+,56+,57+,58+,59+,60+,61+,66+,67?,68+,69+,70-/m1/s1
InChI 密鑰
QJDWKBINWOWJNZ-IDGBIKHQSA-N
Amino Acid Sequence
一般說明
應用
生化/生理作用
Cinnamycin, like other lantibiotics, was also reported to inhibit phospholipase A2 (PLA2). It was suggested as an alternative treatment for atherosclerosis through its ability to inhibit PLA2 by binding to its substrate PE. Moreover, Cinnamycin was found to inhibit Herpes simplex virus (HSV-1) activity.
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
分析證明 (COA)
輸入產品批次/批號來搜索 分析證明 (COA)。在產品’s標籤上找到批次和批號,寫有 ‘Lot’或‘Batch’.。
客戶也查看了
文章
Ribosomally synthesized antimicrobial peptides are a promising focus in antibiotic research amidst bacterial resistance and emerging infectious diseases.
在細菌抗藥性和新興傳染性疾病的情況下,核糖體合成的抗菌肽是抗生素研究中一個很有前景的焦點。
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務