HPLC Troubleshooting Guide
How to Identify, Isolate, and Correct the Most Common HPLC Problems
Although HPLC method development has been improved by advances in column technology and instrumentation, problems still arise. Every troubleshooting guide mentions the reproducibility of selectivity as the most important column criterion; hence, most manufacturers have done their best to validate their production processes. Nevertheless, small differences that exist in surface chemistry tend to appear at the analysis of very sensitive samples. It is virtually impossible to eliminate all these during production in view of the variables involved: crude materials and reagents, surface binding chemistry, the packing process itself as well as the column packer, laboratory equipment, and environment. In addition to this, the surface chemistry tends to change during column use. The bound phase is disintegrated, silica dissolves as silicate, and the extended surface tends to adsorb impurities from the sample and mobile phase. Therefore, these small differences have to be compensated for by the ruggedness of the method.
In this guide, we offer you a systematic means of isolating, identifying, and correcting many typical HPLC problems.
The most important thing is to keep in mind four simple main rules:
- The rule of “one”: never change more than one thing at a time. Making several changes makes it difficult to speculate from which change effect comes.
- The rule of “two”: any effect or problem must be repeatable to be able to troubleshoot it. If you cannot repeat a chromatographic problem, then it is very difficult to find out what is causing it and how to fix it.
- "Put it back”: after all, often problems can be avoided by routine maintenance (e.g., planned replacement of worn-out parts) on a regular basis, keeping good records of what has been done and when.
- Keeping accurate records: Most problems do not occur overnight but develop gradually. Accurate record keeping is vital to detecting and solving many problems.
Evaluate every column you receive when you receive it and at regular intervals thereafter. By keeping a written history of column efficiency, mobile phases used, lamp current, pump performance, etc., you can monitor your system’s performance.
Records also help prevent mistakes, such as introducing water into a silica column, or precipitating buffer in the system by adding too much organic solvent. Many analysts modify their HPLC systems in some way. Reliable records are the best way to ensure that a modification does not introduce problems. For problems relating to pumps, detectors, automatic samplers, and data systems, consult your instrument manual’s troubleshooting guide or contact your instrument service engineer.
The important segments of an HPLC system are the same, whether you use a modular system or a more sophisticated unit. Problems affecting overall system performance can arise in each component. Some common problems and issues that may appear and how to solve them are discussed here. Chromatographers frequently have to identify and rectify problems, which can be divided into different categories. Solutions to these problems are presented in easy-to-use tables.
ISOLATING HPLC PROBLEMS
In an HPLC system, problems can arise from many sources. First, define the problem, then isolate the source.
Determine which component(s) may be causing the trouble. A process of elimination will usually enable you to pinpoint the specific cause and correct the problem.
Want to transfer your HPLC method to a UHPLC method? Get help calculating the run-time and solvent-consumption savings from transferring methods using our HPLC Method Transfer Calculator.
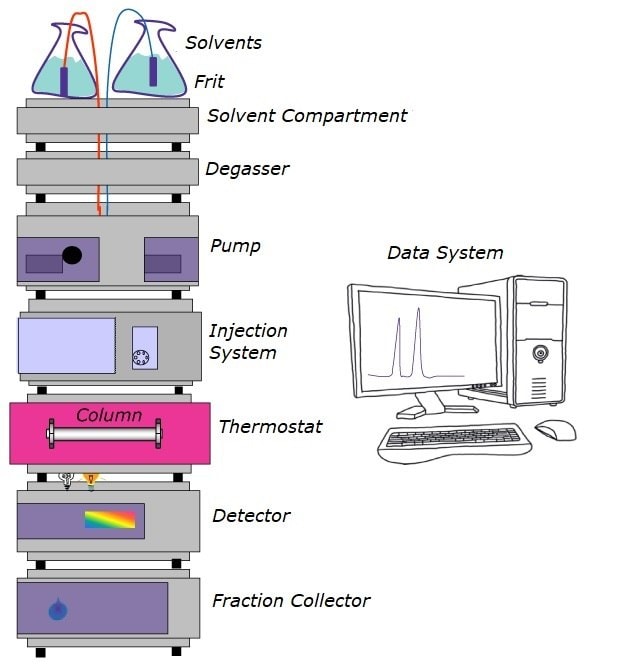
Figure 1.Components of an HPLC System
HOW TO PREVENT MOBILE PHASE PROBLEMS
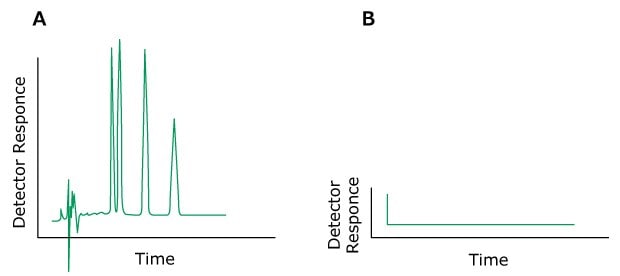
The most important part of a chromatographic setup is the column. But even though it provides retention, the final separation also depends strongly on the mobile phase (MP). The various effects offered by the mobile phase influence the retention and differential migration (selectivity) of the solutes through the column. Low sensitivity and rising baselines, noise, or spikes on the chromatogram can often be attributed to the contamination of the mobile phase. Impurities in the mobile phase are especially troublesome in gradient elution, as the accumulation of even small impurities might be happening over time, “waiting” for the increased elution power of the mobile phase to elute. The baseline may rise, and spurious peaks can appear as the level of the contaminated component increases.
Often, problems with chromatographic separation are related to an incorrectly/inconsistently prepared mobile phase. Hence, an inclination to use simpler mobile phases can be observed for practical reasons of increased method robustness, easier method transfer, and ease of use (e.g. in clinical diagnostics or in process control).
Solvent/Mobile Phase Additive Purity
Most of you are familiar with the rule: “garbage in, garbage out.” This adage is especially true when selecting the correct purity of mobile phase components. For example, it is an absolute must to use gradient-grade solvents and reagents for gradient separations to get an accurate, reproducible, and clean baseline (free of ghost peaks), and sensitive chromatographic separation. Using the correct and suitable grade of solvents based on the application method (e.g., hypergrade for LC-MS, see more at SigmaAldrich.com/solvents) also minimizes the chances of contamination and extends the longevity of a chromatographic column.
Water is the most common source of contamination in reversed phase analyses. You should use only high-purity water, HPLC or LC-MS grade depending on your analyses. It can be commercially available bottled water or ultrapure water from a suitable Milli-Q® water purification system. Ultrapure Milli-Q® systems are an excellent choice for chromatographers as they offer a unique combination of optimized water purification, including a final filter containing reverse-phase C18 silica, and monitoring technologies. The selection of the optimal ultrapure (Type 1) water system for your laboratory will depend on several parameters, such as: feed water quality available, daily volume needs, monitoring requirements, certification levels expected, and any other specific requirements you may have. If you use distilled or deionized (DI) water, keep in mind that common deionizers may introduce organic contaminants into the water, putting your analyses at risk.
Use only HPLC (or LC-MS) grade solvents, salts, ion pair reagents, and base and acid modifiers. Cleaning lower quality solvents is time consuming, and trace levels of contaminants often remain. These trace contaminants can cause problems when you use a high-sensitivity ultraviolet, fluorescence, or mass spectrometry (MS) detector. If you use mass spectrometry as final detector, use MS grade solvents and additives to ensure highest overall method sensitivity and avoid MS instrument contamination.
Because many aqueous buffers promote the growth of bacteria or algae, you should prepare these solutions fresh, and filter them using 0.22 μm membrane filter if you perform UHPLC analysis or 0.45 μm filter for standard HPLC analysis before use. Filtering also will remove particles that could produce a noisy baseline or plug the column. Prevent microorganism growth by adding about 100 ppm sodium azide to aqueous buffers. Alternatively, these buffers may also be mixed with 20% or more of an organic solvent such as ethanol, acetonitrile, methanol or isopropanol.
For cases requiring addition of any reagent, like buffer, it is to be ensured that the reagent meets the required quality and has not passed its expiration date. The degradants from expired additives lead to ghost peaks in sample chromatograms. Certain additives degrade quicker, depending on their nature (for example, 20 mM, pH 7 phosphate buffer). Improper/careless handling (for example, left over solvent put back into bottle, bottle left on the lab bench without the cap closed, lost pipette tips floating inside the bottle and so on) of these reagents spoils chemicals quickly.
Mixing mobile phases
For isocratic separations with premixed mobile phases, solvent volumetric contractions in commonly used mixtures (water/acetonitrile, methanol, or tetrahydrofuran) should be taken into account during their preparation. The only correct way to prepare such mobile phase mixtures is to separately take precisely measured volumes of the components and mix them. For example, to get a 70% organic mobile phase, 300 mL of water and 700 mL of organic solvent should be precisely measured separately and then combined together in a flask. But if only the water is measured precisely and the organic solvent is then added to make up the required final volume, due to the solvent mixture contraction, the resulting solvent strength will be a little higher (or weaker in case organic solvent was added first and water was added later). For premixing of MPs, attention should be taken to the toxic solvent fumes that might be emitted, under a fume hood.
Nowadays, gradients are generally correctly formulated using gradient pumps; however, some minor differences in retention behavior might be observed during comparison of the instruments with low- pressure and high-pressure gradient systems due to their mixing mechanisms. Bot approaches have pros and cons – low pressure gradient formation instruments are simpler and, therefore easier to maintain, but the main advantage is constant flow independent from the gradient. The flow obtained from high pressure gradient formation systems is a few percent lower as set, because of the solvent contraction after mixing. This phenomenon needs to be kept in mind if someone is transferring an established method from one type of instrument to the other.
In Reversed-Phase chromatography, most of the time it is better to work with premixed mobile phases such as 5% acetonitrile in water and/or 5% water in acetonitrile. The rationale behind such preference is to increase degassing effectiveness, avoid the mixture heating (e.g. methanol in water) or cooling (e.g., acetonitrile in water) upon mixing, and also to improve mixing efficiency by making the two mobile phases more similar in viscosity and surface tension. The limitations of such premixed solvents are that the solvent strength of mobile phase B cannot be 100% and that this is an extra step in mobile phase preparation– which is an additional, potential source of error. Typically, it is recommended to use mobile phase solvents directly out of their delivery containers to prevent additional chances of contamination.
When analyzing samples containing ionizable compounds, the buffer can be one of the most important variables controlling retention in an HPLC separation. The pH of the mobile phase determines the presence of ionizable compounds (analytes and matrix) in either an ionized or non-ionized state. The ionized species in reverse phase (RP) chromatography always elute from the column earlier than the non-ionized species. Changing the pH can also increase the selectivity for effective separation of closely eluting or overlapping peaks. Run-to-run variability in pH results in separation inconsistency. Buffers prevent pH variations. Therefore, the proper buffer choice, in terms of buffering species, ionic strength, and pH, is the most critical step in the development of an HPLC method when ionizable substances are analyzed.
Tips for choosing LC buffer
Buffer selection. The choice of the appropriate buffer for an application is governed by buffer characteristics such as pKa, pH range, and UV cut-off. As a rule, the buffers should be used for a pH within +/- 1 unit of their pKa value. Within this range, the buffers resist any deliberate attempts to change in pH. The buffer’s capacity is at its maximum when the pH of the buffer is equal to its pKa. The UV cut-off value also needs to be considered, as the detection wavelength should not interfere with the buffer absorbance (significant absorbance: trifluoracetic acid < 220 nm; formic acid, acetic acid <240 nm). For best results with an ionizable analyte of interest, use a buffer with a pH at least 2 units away from the pKa. If the pH of the mobile phase is too close to the analyte’s pKa, split peaks or shoulders might be observed due to the presence of both species in the sample. For several ionizable analytes of interest, it is preferable to choose a pH value wherein all the analytes exist in the same form, either ionized or non-ionized.
- Measuring buffer pH. The pH of the buffer is the pH of the aqueous portion before the organic mobile phase is added. The addition of an organic solvent normally shifts the pH either up or down (pH shift should be consistent for the same buffer). It is not so important to know the exact pH value of the buffer in an organic medium, but it is important to have a consistent pH value (because the pKa of your analytes is also determined in the aqueous phase, and we do not know the individual pKa shifts either).
- Chemical Purity. The quality/purity of mobile phase additives (buffers, salts, acids, and bases) along with organic solvents utilized in an HPLC experiment must be adapted to the detector sensitivity and elution protocol.
- Chemical Compatibility. Buffer composition, along with the mobile phase pH, must be chosen in agreement with the column housing material and nature of the stationary phase to prevent corrosion of either.
- MS compatibility. Introducing mineral salts into a mass spectrometry system is not advisable. Examples of suitable volatile buffers are ammonium acetate, ammonium formate, and ammonium citrate. pH modifiers like formic acid and acetic acid should be used to control pH and help ionization for LC-MS.
- Buffer Solubility. Ideally, the buffer should be completely water-soluble (RP methods) and should not precipitate during the analysis when mixed with a chosen organic solvent. Buffer concentration must therefore be carefully chosen to avoid precipitation at higher concentrations in the organic solvent. If neglected, this can create operational problems with the pumps and induce HPLC column blockage or backpressure rise.
- Buffer Strength. An eluent showing weak interaction with the stationary phase is only capable of eluting weakly bonded analytes from the column, whereas a strong interaction causes elution of strongly bonded sample molecules. The elution or solvent strength of various solvents depends on the type of stationary phase used. After the elution strength, the viscosity of the buffer plays an important role in terms of its suitability for use in HPLC analyses.
- Buffer Concentration. Ideally, the lowest concentration that gives reproducible results should be chosen. Higher concentrations lead to a faster elution of polar molecules. Generally, the buffer concentration should not be lower than 5 mM. Below this concentration, it may not perform as a buffer (depends on analyte concentration and buffering capability). Raising the buffer concentration can increase viscosity, which in turn can increase column back pressure. Commonly, the concentration should be kept in the 5 to 100 mM range. A concentration higher than 100 mM of mineral salt buffers wear out the pump’s movable parts faster, therefore a back-seal wash is recommended to be installed.
It can be observed that buffers play a crucial role in a majority of HPLC separations. Method developments often require careful selection of buffers and adequate care in their preparation. So, the general rules kept to be in mind are― buffer solutions must be homogeneous, clear, and free from any particles. If stored, please keep in mind that buffers have a limited lifetime, so consider their preparation daily.
ISOLATING PUMP PROBLEMS
The pump must deliver a constant flow of solvent to the column over a wide range of conditions. HPLC pumps incorporate single or dual piston, syringe, or diaphragm pump designs. These pumps may be low or high pressure gradient formation systems (Figures 2 & 3). Both approaches are used; however, the high-pressure gradient pump might deliver a flow rate up to 4% lower than the set value because of solvent contraction after mixing, potentially affecting the precision of chromatographic separations. On the other hand, the low-pressure gradient formation pump delays gradient arrival to the column, introducing challenges in maintaining precise elution conditions particularly pronounced using high speed steep gradients. These operational characteristics should be carefully considered when selecting a pump system based on the specific requirements of the chromatographic analysis, particularly when transferring a method from one instrument to another.
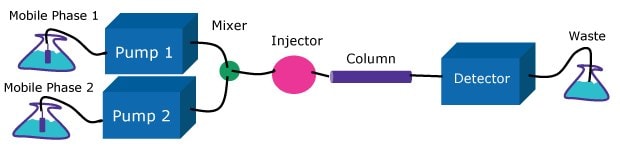
Figure 2High-pressure gradient system
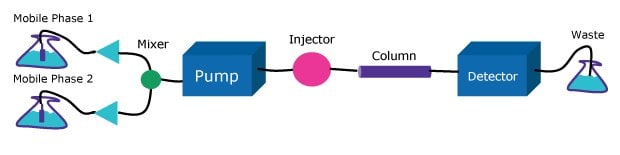
Figure 3.Low-pressure gradient system
Chromatographic pump problems can include various issues that may affect the performance and reliability of the pump in chromatography.
Addressing these problems often involves routine maintenance, checking and replacing consumables, and ensuring proper calibration and operation of the chromatographic pump. Regular system checks and adherence to recommended maintenance schedules are essential for optimal chromatographic performance.
Pumping system problems are usually easy to spot and correct. What is characteristic of pumping system problems is repeating baseline noise, the frequency of which increases proportionally with the flow rate. A sure sign of a leak is a buildup of salts at a pump connection. Buffer salts should be flushed from the system daily with fresh, deionized water. To isolate and repair specific problems related to your apparatus, use the troubleshooting and maintenance sections of the operation manual or contact your instrument vendor. Pump seals require periodic replacement. A preventative maintenance program is highly recommended when looking into purchasing a new (U)HPLC system.
INJECTOR AND INJECTION SOLVENTS
The injector rapidly introduces the sample into the system with minimal disruption of the solvent flow. HPLC systems currently use variable loop, fixed loop, and syringe-type injectors. These set-ups are activated manually, pneumatically, or electrically.
Mechanical problems involving the injector (e.g., leaks, plugged capillary tubing, worn seals) are easy to spot, and correcting them involves addressing various issues that may impact the quality and reproducibility of chromatographic results. Use a precolumn filter to prevent plugging of the column frit due to the physical degradation of the injector seal. Other problems, such as irreproducible injections, are more difficult to solve.
Variable peak heights, split peaks, and broad peaks can be caused by incompletely filled sample loops, incompatibility of the injection solvent with the mobile phase, or poor sample solubility. Whenever possible, dissolve and inject samples in the mobile phase, which flows through the column at the moment of injection. Otherwise, be sure the injection solvent is of lower eluotropic strength than the mobile phase. Be aware that some autosamplers use separate syringe washing solutions. Make sure that the wash solution is compatible with and weaker than the mobile phase. This fact is especially important when switching between reversed and normal phase or Hydrophilic Interaction Liquid Chromatography (HILIC) analyses. Ensuring the cleanliness of the injection needle's external surface is crucial as well, particularly when dealing with high-concentration or viscous samples. External washing of the needle is recommended to prevent any residue from previous injections and avoid contamination of the injection needle set port, which could compromise the accuracy of subsequent analyses and cause cross-contamination between different samples. This can be achieved by immersing the needle in one or more vials containing wash solutions designed to thoroughly clean the exterior of the needle. Proper external washing not only helps maintain the integrity of the chromatographic system but ultimately contributes to the precision and reliability of analytical results.
Proper sample preparation is a critical aspect of ensuring the accuracy and reliability of chromatographic analyses. It is imperative to filter samples before injection to prevent potential clogging of the injector needle and subsequent system issues. Filtering removes particulate matter that could obstruct the narrow channels of the injection needle, ensuring a smooth and consistent injection process. Additionally, when withdrawing samples from vials, it is advisable to avoid injecting from the vial bottom, as sediment may accumulate over time. Sedimentation can lead to the introduction of unwanted particles into the chromatographic system, clogging/polluting the column. By adhering to best practices such as filtering samples and avoiding injections from the vial bottom, analysts contribute to the overall reliability and precision of chromatographic results, minimizing the risk of system contamination and ensuring optimal performance.
COLUMN PROTECTION
Although not an integral part of most equipment, mobile phase inlet filters, pre-injector and pre-column filters, and guard columns greatly reduce problems associated with complex separations. We recommend that all samples be filtered through 0.45 μm or 0.2 μm syringe filters.
Inline filters play a pivotal role in chromatographic systems, serving as indispensable components to enhance the quality and longevity of analytical columns. Positioned strategically within the fluidic path, these filters effectively trap particulate matter, debris, or contaminants present in the mobile phase or sample before they reach the chromatographic column. By preventing the entry of unwanted particles, inline filters contribute to the protection and preservation of the column's packing material, thus extending its lifespan and ensuring consistent and reproducible separations. Inline filters are not meant to replace filtration but are more of a backup protection for the column. Inline filter porosity is recommended to be smaller column inlet frits. For example, the frit at the head of the column packed with 5 µm particles is 2 µm, in this case, the inline filter porosity should be ~1-0.5 µm. When the system's back pressure begins to rise, check and, if needed, replace inline filter.
In all cases, we strongly recommend the use of guard columns. The stationary phase in the guard column should be matched to the analytical column so it should trap substances that would irreversibly adsorbed to the analytical column, limiting its life. The guard column acts as a sacrificial element in the chromatographic system. The useful life of these disposable products depends on mobile phase composition, injection volume, sample purity, pH, etc. As these devices become contaminated or plugged with particles, pressure increases and peaks broaden or split.
GETTING THE MOST FROM YOUR ANALYTICAL COLUMN
Regardless of whether the column contains a bonded reversed or normal phase, ion exchange, affinity, hydrophobic interaction, size exclusion, or resin/silica-based packing, the most common problem associated with analytical columns is deterioration. Symptoms of deterioration are poor peak shape, split peaks, shoulders, loss of resolution, decreased retention times, and high back pressure. These symptoms indicate contaminants have accumulated on the frit or column inlet, or there are voids, channels, or a depression in the packing bed.
Deterioration is more evident in higher-efficiency columns. For example, a 3.0 µm packing retained by 0.5 µm frits is more susceptible to plugging than a 5 or 10 µm packing retained by 2.0 µm or larger frits. Proper column protection and sample preparation are essential to getting the most from each column.
Overloading a column can cause poor peak shapes and other problems.
TYPICAL COLUMN CAPACITY (loading, sample injection)
Column capacity depends on many factors, but typical values for total maximum injection volume and sample amounts of analytes on a column are provided in the table below. The maximum injection volume is considered to be not more than 2% of the total column volume, and the typical sample concentration in HPLC is around 1 mg/mL. Please keep in mind that efficiency and resolution normally increase with reduced injection volume.
SOLVING DETECTOR PROBLEMS
Detector problems fall into two categories — electrical and mechanical/optical. For electrical problems, you should contact the instrument manufacturer. Mechanical or optical problems usually can be traced to the flow cell. Issues with detectors include cell contamination, air bubbles, and leakage. These typically result in low sensitivity, drifts, baseline sounds, or spikes in the chromatograms. Certain cells are pressure-sensitive, particularly those found in refractive index detectors. Flow rates or back pressures that exceed the manufacturer’s recommendation will break the cell window. Old or defective lamps as well as incorrect detector rise time, gain, or attenuation will reduce sensitivity and peak height.
HPLC PROBLEMS, CAUSES AND REMEDIES
Small differences in mobile phase composition may cause huge differences in retention time when the column is overloaded and this also changes with temperature. However, even if the mobile phase is buffered and the pump is working properly, the retention times may fluctuate if the pH is too close to the pK of the sample substance. The pH of the mobile phase should therefore be chosen to be at least one pH unit above or below the pK value of the analytes being separated. Retention time drift indicates insufficient column conditioning. With increasing column life, the retention times may shift towards less retentivity, especially if the user is working at acidic pH (≤pH 2). Abrupt changes in retention time are usually due to errors in the system.
Problem: Changing Retention Times

Figure 4.Variable Retention Times. A) Normal, B) Problem, C) Problem
Problem: Decreasing Retention Times
Problem: Increasing Retention Times
Chromatography column equilibration is a crucial step in the chromatographic process, essential for ensuring accurate and reproducible results. This process involves allowing the mobile phase to flow through the column under specific conditions until a stable baseline is achieved, indicating that the system is in equilibrium.
Equilibration serves several important purposes. Firstly, it allows the stationary phase inside the column to interact with the mobile phase, ensuring proper wetting and conditioning of the packing material. This helps to establish a uniform distribution of the mobile phase and analytes within the column, optimizing separation efficiency and resolution.
Additionally, equilibration helps to stabilize system parameters such as pressure, temperature, and flow rate, minimizing variability during subsequent injections. By allowing the system to reach a steady state, equilibration reduces the risk of baseline drift and ensures consistent performance over time.
Problem: Slow column equilibration time
Problem: Varying retention times
Peaks
If all peaks have same appearance in the chromatogram, the problem originated before the separation. If only some of the peaks or only one peak in the chromatogram elute with a distorted shape, the source is of chemical nature.
Problem: Broad Peaks
Wide peaks are generated either by substantial influence on the part of the HPLC system (bad capillary connections, void volumes, too large detector cells or ill-chosen time constants) or by poor column performance.

Figure 5.Typical chromatograms, A). Normal peaks B) Broad peaks
Problem: Ghost Peaks
Ghost peaks may be caused by unknown sample components, late eluting peaks from previous injections, impurities or mixing problems in connection with the mobile phase. The sample should therefore preferably always be dissolved in the eluent or in a solvent with weaker eluting strength. Substances with UV absorption lower than the eluent may generate negative peaks.
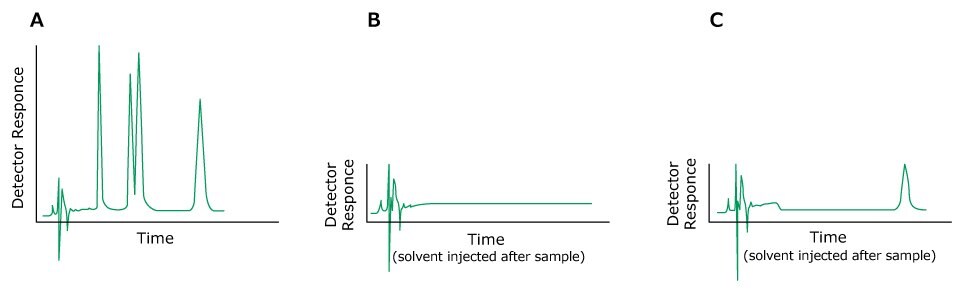
Figure 6.HPLC chromatograms, A) Previous sample, B) After injection of solvent following the solvent with no ghost peak, C) After injection of solvent following the solvent showing a ghost peak
Problem: Negative Peaks

Figure 7.A) Normal chromatogram, B) Chromatogram with negative peaks
Problem: Peak Doubling
If all peaks have shoulders or elute as double peaks, the cause may originate from clogged inline filters, column inlet frits, contaminated pre-columns or a void volume at the column head. In most cases, the column may be returned to its original state by cleaning or replacement of the inlet frit. A short-term solution to this problem may also be to invert the column. Destroyed bed at the column outlet contributes only marginally to peak spreading.
Problem: Peak Fronting
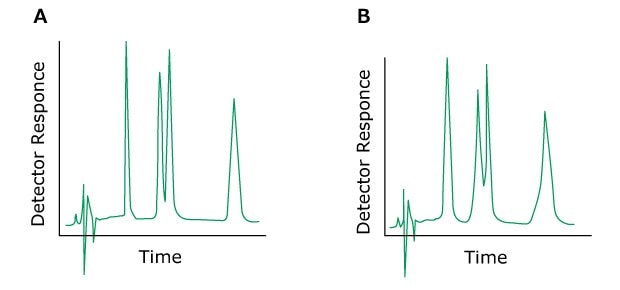
Figure 8.Typical chromatograms, A) normal without a fronting peak B) with a fronting peak
Problem: Peak Tailing
The tailing of peaks that are eluted early is caused by extra column effects. To remedy, the entire system should be checked – capillary connections, tubing’s and the detector cell. Secondary, non-specific interaction with the silica gel surface leads to a tailing of late eluting peaks and even to the appearance of double peaks. Addition of triethylamine or acetate to the mobile phase or selecting a suitable stationary phase will considerably improve the peak form. An inappropriately selected pH-value for the mobile phase may also lead to peak tailing. In principle, chromatography should be carried out one pH unit above or below the pK values of the sample substances.
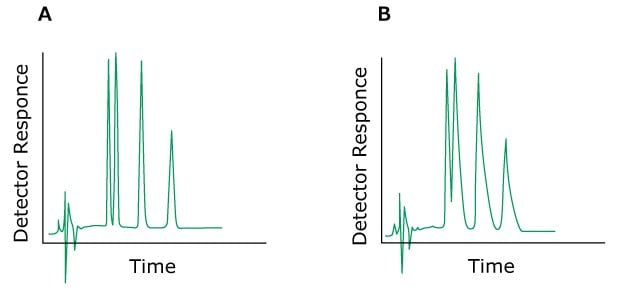
Figure 9.Typical chromatograms, A) Normal without a tailing peak B). With a tailing peak
Problem: Spikes
Problem: No Peaks
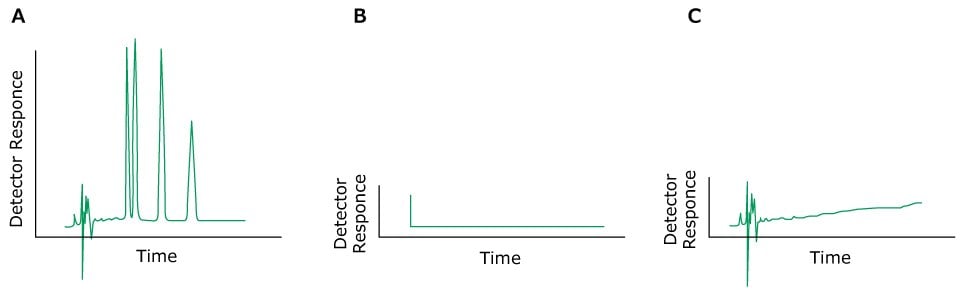
Figure 10.Typical chromatograms, A) Normal peaks, B) No peaks, C) Very small peaks
Problem: Peaks with shoulders, split peaks
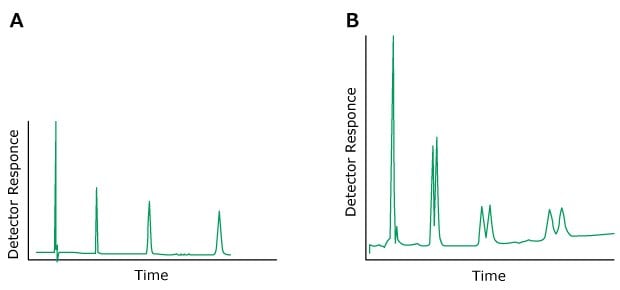
Figure 11.Typical chromatograms A) Normal peaks B) Split peaks
Problem: Change in Peak Height (One or More Peaks)
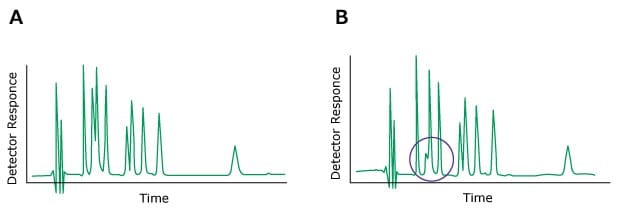
Figure 12.Typical chromatograms, A) Normal B) One peak with a different height
Problem: Loss of Resolution
Problem: Rounded Peaks
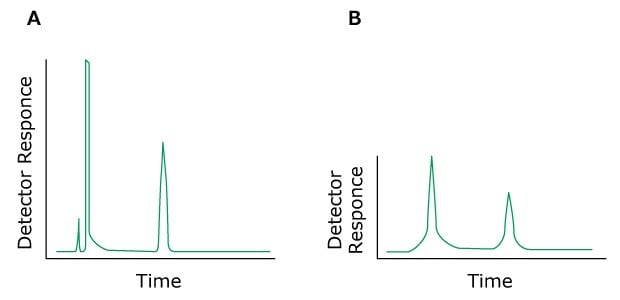
Figure 13.Typical chromatograms, A) Normal, B) Rounded Peaks
Several factors influence sample recovery from the chromatographic column, including column chemistry, mobile phase composition, analyte properties, and operating parameters. The choice of column packing material and stationary phase chemistry can significantly impact analyte retention and elution behavior. Optimizing column selection to match the analyte's physicochemical properties is crucial for minimizing unspecific sorption and maximizing sample recovery. Mobile phase composition plays a critical role in analyte elution and sample recovery. By adjusting the composition and gradient profile of the mobile phase, analysts can enhance analyte elution efficiency and improve analyte recovery.
Problem: Poor Sample Recovery
Often, after new parts have been installed, especially if the installation was not done properly, we may experience some leaks. Additionally, a sudden increase in system back pressure can also cause leakage. Old, wearied out, clogged parts may also be responsible for leakage.
Problem: Leak at Column or Fittings
Problem: Leak at Detector
Problem: Leak at Injection Valve
Problem: Leak at Pump
Sudden variations in chromatographic selectivity can arise from a variety of factors, including column conditioning, mobile phase composition, sample matrix effects, temperature and flow rate fluctuations, and column degradation. By understanding these factors and implementing appropriate measures to mitigate their effects, analysts can maintain the reliability and reproducibility of chromatographic results, ensuring accurate and consistent analytical performance.
Problem: Differences In Selectivity

Figure 14.Typical chromatograms, A) Normal, B) Problem of change in selectivity
The chromatographic baseline serves as the reference point for the detection of analytes in chromatographic analysis. It represents the signal produced by the detector in the absence of any analyte elution, indicating the baseline noise level and system stability. A stable and well-defined baseline is essential for accurate peak detection, integration, and quantification in chromatographic analysis. Variations or disturbances in the baseline, such as drift, noise, or spikes, can obscure analyte peaks and lead to inaccurate results. Factors contributing to baseline disturbances include fluctuations in mobile phase composition, temperature, pressure, and detector sensitivity, as well as instrument-related issues such as detector noise and electronic interference. Proper baseline correction techniques, such as baseline subtraction or filtering, are often employed to enhance signal-to-noise ratio and improve data quality. Additionally, regular system maintenance, including column flushing, detector cleaning, and instrument calibration, is essential for maintaining baseline stability and ensuring the reliability of chromatographic results. In summary, the chromatographic baseline plays a critical role in chromatographic analysis, serving as the foundation for accurate and precise determination of analyte concentrations. Maintaining baseline stability is essential for achieving reliable and reproducible chromatographic results in analytical applications.
Problem: Disturbance at Void Time
Problem: Drifting Baseline
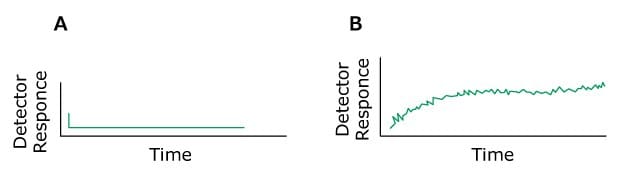
Figure 15.Typical chromatograms, A) Normal B) Baseline Drift
Problem: Noise
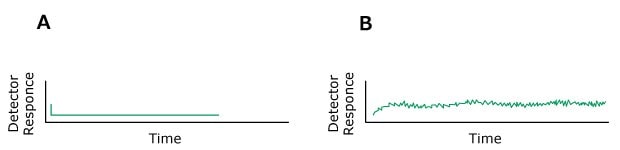
Figure 16.Typical chromatograms, A) Normal, B) Baseline noise (regular)
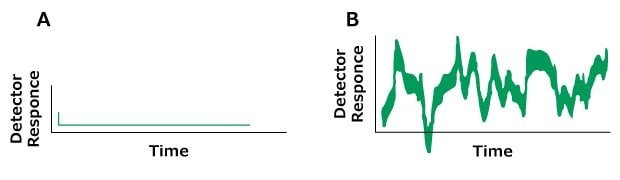
Figure 17.Typical chromatograms, A) Normal B) Problem (Irregular baseline noise)
Problem with pressure is usually connected with too high back-pressure, and to elucidate where the problem is originating, a good laboratory practice is to disconnect the system stepwise starting at the detector and working, sequentially, back to the injector and then to the pump.
Problem: Decreasing pressure
Problem: Fluctuating pressure
Problem: High back-pressure
Further Recommendations
We also suggest referring to the maintenance and troubleshooting sections of your instrument manual or contacting your service engineer. Modern HPLC systems often have self-diagnostic capabilities that help isolate the problem area within the instrument. For persistent problems relating to the column or your analysis, please contact Supelco’s Technical Service Department.
The remaining pages in this guide include procedures for restoring column performance following loss in resolution, retention, or selectivity, suggestions on how to prevent and solve column hardware problems, and a selection of column protection products from the Supelco portfolio. Please refer to our website for our complete line of accessories that prolong column life and, in general, simplify or improve your (U)HPLC or FPLC® analysis
RESTORING YOUR COLUMN’S PERFORMANCE
Exposure of a column to samples or solvents containing highly adsorptive components will result in increased backpressure and a change in selectivity. Often, the column can be restored to its original performance by following suitable wash protocols. When performing solvent rinse regeneration, the column should be reversed and transferred from the analytical HPLC system to a simple, inexpensive pump. Alternatively, disconnect the column from the detector and rinse directly to waste. Each solvent should be rinsed with a minimum of 20, preferably 30, column volumes.
It is critical to carefully read the instruction sheet that comes with each column first. This sheet also contains information about suggested column care and regeneration procedures. These recommendations must be followed. Column contamination at the top by particles generally should be easily removed by washing the column for about 5 to 20 column volumes in a backflush mode, but only if the column manufacturer allows it – important to make sure that both column frits (inlet and outlet) have the same porosity. Please keep in mind, that small particles trapped deeper in the column packing are unlikely to be removed. As a result of such a washing, the backpressure in the column should be somewhat lower. To remove unspecifically bound material from the column is difficult, and the outcome is usually unpredictable because normally we do not know what compounds the contaminants are. The success ratio might range from 0 to 100 %. To fully restore column packing, uniformity for columns packed with particles smaller than 5 µm is very unlikely. Normally, column backflush only helps for a short time.
If you decide to flush/regenerate your column, the following step by step procedures should theoretically rejuvenate a column whose performance has deteriorated due to sample contamination.
Note! All solvents used for column regeneration must be the same analytical quality level as normally used with this column performing daily analysis.
- Disconnect and reverse the column.
- Connect it to the pump, but not the detector.
- Follow the appropriate flushing procedure in the table provided below, using a flow rate that results in column back pressure of 1500-4500 psi, but never higher than the maximum recommended pressure in the manufacturer’s instruction manual. If you have a Supelco® column, analyze with the test mix and the conditions listed on the data sheet.
- Condition your column with the usual solvent
- Test column using test mixture sample. Efficiency, symmetry, and capacity should be within 10-15% of the test sheet values. If not, replace the column.
Note: Volumes listed in the table are for 25 cm x 4.6 mm I.D. columns, which have a column volume of 4.15 mL. When restoring a 4.6 mm I.D. column shorter or longer than 25 cm, multiply all volumes by the ratio of the column length to 25 (e.g., for a 15 cm column: 15/25, or 0.6 times the volume). When restoring a column of internal diameter other than 4.6 mm, multiply all volumes by the ratio of the square of the column I.D. to (4.6)2 (e.g., for a 3.2 mm I.D. column: (3.2)2/(4.6)2 = 10.24/21.16 = 0.48 times the values in the table).
Column Restoration Procedures |
|---|
Properties of typical organic solvents commonly used in HPLC |
|---|
Nonbonded Silica Columns Exposed to Polar Solvent
Samples and mobile phases containing very strongly polar solvents, such as water or alcohols, can deactivate uncoated silica HPLC columns. This action can drastically affect column performance, particularly solute retention and selectivity. Even prolonged column flushing with a nonpolar solvent only partially restores column performance, while wasting chemicals. A silica regeneration solution (containing acetic acid, dimethoxypropane, and methylene chloride) can quickly and inexpensively restores silica column performance by removing trapped polar material. Pump the solution through the affected column for 10 minutes at a rate of 4 mL/minute, then flush with mobile phase for 10 minutes at a rate of 2 mL/minute. Evaluate column performance by using a test mixture for evaluating silica columns. Performance should be virtually the same as before the polar solvent was introduced.
STORING THE COLUMN
Column storage may be short, middle and long term.
- Short term storage, i.e. overnight, the mobile phase used in last analysis can remain within in the column. Alternatively, a passage of the mobile phase at very low flow rate is also possible (especially if buffer concentration in the mobile phase is high, >50 mM). In this case column conditioning potentially can be skipped before continuing the analysis next day. This option is particularly recommended for normal phase separations, where change in mobile phase composition can result in lengthy re-equilibration. However, if buffer concentration in the mobile phase is very high (>0.5 M) then pump part's life time (e.g. injector & switching valves) could depend on the time of contact with high concentration buffer. Same is true for the column if pH is close to the limit of the column (for most of silica based columns pH 2 to pH 7). Some salts, like chloride salts in particular, are very corrosive to stainless steel, and might attack the column wall as well as inlet–outlet frits. In such cases, column (and all system) should be flushed to less harsh mobile phase. In this case, I would recommend rinsing the column with water rich mobile phase (~90%) with about 10 column volumes (approximate column volumes for some popular column dimensions can be found in the table below).
Approximate Column Volumes for some Popular Column Dimensions and Multiples of it |
|---|
If you disconnect a column from the instrument, end plugs should be tightly fitted to prevent solvent evaporation leading to drying of stationary phase. The worst-case scenario is an improperly washed column previously used with high concentration salt and dried out over time – salt crystals will be formed, the column most probably will be irreversibly damaged. However, some columns might can be stored dry some not, please check the manufacturer’s column care guidelines. Standard HPLC columns should be stored at room temperature and never in a refrigerator or freezer (exceptions are protein modified affinity or active enzyme reactor columns). This paragraph’s recommendations are valid for mid- and long-term column storage too.
- Medium interval storage, i.e. 2 days or over the weekend, columns should be flushed. Flush intensity or volume depends on the buffer concertation used during analysis. It is generally advisable to first flush buffering agents off the column with about 10 column volumes of mobile phase with 10% organic solvent in water. In this case washing will be effective also we would avoid buffer precipitation and possible column dewetting problems. Then the column might be left connected to the instrument or disconnected and closed with end plugs. Please consider short term column storage advises too, e.g. referencing column documentation for recommended storage solvent.
Storing a HILIC column in an acetonitrile–water mixture may take a long time to re-equilibrate if a low ionic strength buffer (for example, 5 mM ammonium acetate) is used for the analytical method. Therefore for HILIC columns it is recommend to store these in solvents containing 80–90% acetonitrile and buffers containing 5–10 mM ammonium acetate or ammonium formate.
Ion-exchange and mixed-mode phases containing carboxylic acid functional groups (for example, weak cation-exchange phases) cannot be stored in solutions containing alcohols, because of slow esterification and the resulting change in selectivity/capacity.
- For long term storage, silica based columns, after proper washing with min. 15 column volumes using ~ 10% organic solvent in water, should be flushed with organic rich mobile phase for min. 10 column volumes and might be stored in an aprotic solvent. If water might also be present, it should not be in higher concentrations, definitley less than 50%. The best storing solvent advised in the literature is acetonitrile or methanol (some exceptions exists, for example columns with amide modification, these should be stored in acetonitrile only). Some studies1 also indicate that at RP conditions rates of erosion and corrosion of the stainless steel components of the HPLC using pure acetonitrile or methanol were higher compared to when they were mixed with water. Therefore, 90 % acetonitrile or methanol is a perfect long-term storage agent for most reverse phase columns. For reverse phase columns storage solution mixture of isopropanol and water (80/20), because of isopropanol’s higher viscosity & vapor pressure and the reduced chance for column dry out, even if end fittings are not completely sealed. Isopropanol is also a stronger eluent, therefore; after storing in isopropanol, we could be sure that even more impurities will be washed out than with acetonitrile or methanol gradients. Last but, not least isopropanol is also less toxic. Important to note that all mobile phases used for flushing, washing or column storing must be of the same quality grade as the ones used for the analysis. Columns should be stored at room temperature (exceptions might be e.g. affinity columns as mentioned before) in their original box, with a copy of the certificate of analysis (CoA)/Column Report, maybe also with column log book to see previous uses, to evaluate the column prior to future use.
How long columns be stored? This depends on many aspects. Some columns do not change even after 5 or 10 years of storage. If you decide to try a column after such a long storage, assume that the column most probably had dried out, and needs to be rewetted by first flushing with 100% acetonitrile (RP-phases), and then equilibrating in mobile phase for about 1 h before making any selectivity measurements. Additionally, maybe run a column test mix and compare the data to the CoA.
Correct column storage is essential for proper chromatography and prolonged column lifetime. Last but not least - always follow the manufacturer’s guidelines for column operational details!
Column Test Mixes
Performance evaluation mixes for HPLC columns.
Well defined test mixes enable you to troubleshoot chromatographic problems, optimize system efficiency, and evaluate columns under conditions where their performance is understood. We ship our test mixes in amber ampuls to prevent photodegradation, and we include instructions for proper use and interpretation of results.
PREVENTING AND SOLVING COMMON HARDWARE PROBLEMS
Preventing Leaks
Leaks are a common problem in HPLC analyses. To minimize leaks in your system, avoid interchanging hardware and fittings from different manufacturers. Incompatible fittings can be forced to fit initially, but the separation may show problems and repeated connections may eventually cause the fitting to leak. If interchanging is necessary, use appropriate adapters and check all connections for leaks before proceeding.
Highly concentrated salts (>0.2 M) and caustic mobile phases can reduce pump seal efficiency. The lifetime of injector rotor seals also depends on mobile phase conditions, particularly operation at high pH. In some cases, prolonged use of ion pair reagents has a lubricating effect on pump pistons that may produce small leaks at the seal. Some seals do not perform well with certain solvents. Before using a pump under adverse conditions, read the instrument manufacturer’s specifications or contact your service engineer. To replace seals, refer to the maintenance section of the pump manual, or, again, contact your service engineer.
Unclogging the Column Frit
A clogged column frit is another common HPLC problem. To minimize this problem from the start, use a precolumn filter and guard column. To clean the inlet, first disconnect and reverse the column. Connect it to the pump (but not to the detector), and pump solvent through at twice the standard flow rate. About 5-10 column volumes of solvent should be sufficient to dislodge small amounts of particulate material on the inlet frit. Evaluate the performance of the cleaned column using a standard test mixture.
A SELECTION OF COLUMN PROTECTION PRODUCTS
Supelco® Mobile Phase Filtration Apparatus (connect to aspiration line)
- Filtration Apparatus 1 (connects to 1000 mL sidearm flask): Includes 250 mL glass reservoir, funnel base and stopper, clamp, stainless steel holder and screen, 10 Teflon gaskets, 50 Nylon 66 filters (47 mm, 0.45 μm pores).
- Filtration Apparatus 2 (connects to aspiration line): Includes 250 mL glass reservoir, 34/45 tapered funnel base, 34/45 tapered 1000 mL flask and glass cap, clamp, stainless steel holder and screen, 10 Teflon gaskets, 50 Nylon 66 filters (47 mm, 0.45 μm pores).
Protect your instrument and columns by removing particles and gases from solvents and other mobile phase components. Nylon 66 membrane filters are compatible with all solvents commonly used in HPLC.
Filters
A pre-column filter is essential for protecting HPLC columns against particulate matter which can accumulate on the column frit, leading to split peaks and high back pressure. Sources of particles include mobile phases (especially when buffers are mixed with organic solvents), pump and injector seals, and samples. Use a 2.0 μm frit to protect columns containing 5 μm or larger particles, or a 0.5 μm frit for columns with particles smaller than 5 μm.

Our Supelco® pre-column filter can be connected directly, hand-tight, into any HPLC column or guard column listed on our website, or with any other column that has Valco-compatible end fittings. PEEK cap and body, 2 μm stainless steel frit.
Valco Precolumn Frit and Screen Filters3
In-line installation. Efficient, low dead volume filters protect your columns from particles without reducing column performance. The replaceable 1/8" frit has 0.5 μm pores to protect 3 μm or 5 μm column packings, the replaceable screen has 2 μm pores. Choose the frit filter for higher filtration capacity (most applications) or the screen filter for less dead volume (e.g., with microbore columns). Use with 1/16" O.D. tubing; 1/16" fittings included.

Isolation Technologies Pre-column Filter
In-line installation. High-capacity inlet filter minimizes dead volume and band broadening, to prevent loss of column efficiency while protecting your column. Frit porosity: 0.5 μm. Complete as shown.

SSI High Pressure Pre-column Filter
In-line installation. The 316 stainless steel filter disc (0.5 μm pores) is easily replaced without removing the column end fitting. Maximum operating pressure: 15,000 psi (1054 kg/cm²). For 1/16" tubing.

SSI High Pressure Pre-injector Filter
Place between the pump and injector to provide final filtration for the mobile phase. Easily replaced 316 stainless steel filter element (0.5 μm pores). Maximum operating pressure: 15,000 psi (105 MPa). For 1/16" O.D. tubing, 10-32 threads

Upchurch Pre-column Filter
In-line installation. Stainless steel body with inert polyetheretherketone (PEEK) end fittings and a 0.5 μm or 2 μm PEEK frit in one end fitting.

Rheodyne Model 7725 and 7725i Injectors
The Rheodyne Model 7725 injector allows you to inject 1 μL-5 mL samples with accuracy and precision. The rugged, easily maintained design offers many advanced features:
- Patented, continuous flow design (see figure) – flow is uninterrupted when you switch from LOAD to INJECT
- Easy seal adjustment using pressure screw on front of injector.
- Wide port angle (30 °), for easy access to fittings
Injector includes a 20 μL sample loop and is supplied with a VESPEL rotor seal that can be replaced with a Tefzel rotor seal for operation at pH 0-14. Factory set at 5000 psi (345 bar), adjustable to 7000 psi (483 bar). Model 7725i has an internal position sensing switch.
A conventional HPLC valve momentarily interrupts flow during sample injection, subjecting your column to repetitive pressure shocks. Rheodyne’s patented MBB (make-before-break) design makes the new connection before breaking the old one, providing uninterrupted flow.

Rheodyne Model 7725 injector. 1. To sample loop, 2 From pump, 3. MBB passage, 4. MBB port, 5. To column, 6. From sample loop
Optimize Technologies Pump Replacement Parts
A preventative maintenance program that includes routine replacement of pump parts that are subject to wear will help you avoid costly downtime. Our extensive selection of Optimize Technologies check valves, seals, and pistons meet or exceed pump manufacturers' specifications. For the most up-to-date selection of pump parts, refer to the website.

SSI™ LO-Pulse™ Damper
A pulse damper controls pump pulsations for a more stable baseline. The SSI™ LO-Pulse™ damper is a patented unit compatible with single piston reciprocating HPLC pumps (Altex 110A, Eldex pumps, LDC Mini-Pump VS, SSI Models 200 and 300, etc.). At pressures from 500 psi to 6000 psi (35-420 kg/cm²), it improves precision of quantitative analyses and detection limits for trace sample components. Fittings and instructions included.
To continue reading please sign in or create an account.
Don't Have An Account?