F0162
Fibronectin Proteolytic Fragment from human plasma
lyophilized powder, 45 kDa
Synonym(s):
Fibronectin
About This Item
Recommended Products
biological source
human plasma
Quality Level
Assay
≥90% (SDS-PAGE)
form
lyophilized powder
mol wt
45 kDa
packaging
pkg of 0.5 mg
technique(s)
cell culture | mammalian: suitable
impurities
HIV and HBsAg, source material tested negative
Small proteolytic fragments, may contain traces
solubility
water: soluble ≥0.500 mg/mL, clear to slightly hazy, colorless
UniProt accession no.
shipped in
wet ice
storage temp.
−20°C
Gene Information
human ... FN1(2335)
Looking for similar products? Visit Product Comparison Guide
General description
Application
Biochem/physiol Actions
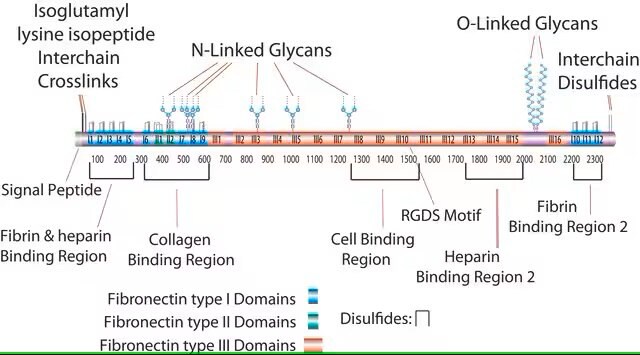
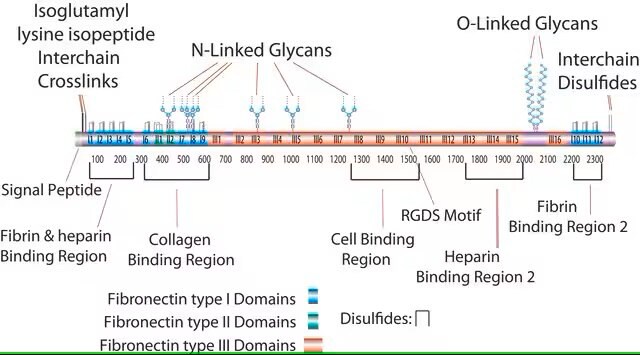
This fragment has an acidic pI (4.9-5.3) and does not bind to heparin. This domain is resistant to proteolysis due to intrachain disulfide bonding and the attached carbohydrate. The intrachain disulfide bonds are essential for binding to gelatin, while the complex, branched, asparagine-linked carbohydrate is not. This fragment binds to C1q, but not to fibrin.
Caution
Preparation Note
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Protocols
Dilute fibronectin for cell attachment, varying per cell type. Coating protocol, products, and FAQs provided.
Dilute fibronectin for cell attachment, varying per cell type. Coating protocol, products, and FAQs provided.
Dilute fibronectin for cell attachment, varying per cell type. Coating protocol, products, and FAQs provided.
Dilute fibronectin for cell attachment, varying per cell type. Coating protocol, products, and FAQs provided.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service