90081
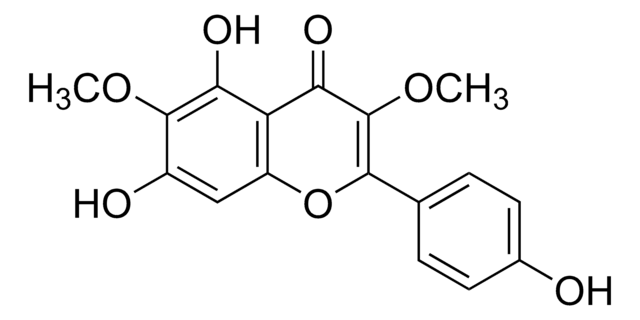
3-O-Methylquercetin
≥97% (HPLC)
Synonym(s):
5,7,3′ ,4′ -Tetrahydroxy-3-methoxyflavone, Quercetin 3-O-methyl ether
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C16H12O7
CAS Number:
Molecular Weight:
316.26
Beilstein:
324509
MDL number:
UNSPSC Code:
12352202
PubChem Substance ID:
NACRES:
NA.32
Recommended Products
Quality Level
Assay
≥97% (HPLC)
form
powder
SMILES string
COC1=C(Oc2cc(O)cc(O)c2C1=O)c3ccc(O)c(O)c3
InChI
1S/C16H12O7/c1-22-16-14(21)13-11(20)5-8(17)6-12(13)23-15(16)7-2-3-9(18)10(19)4-7/h2-6,17-20H,1H3
InChI key
WEPBGSIAWZTEJR-UHFFFAOYSA-N
Biochem/physiol Actions
3-O-Methylquercetin significantly inhibits cyclic adenosine monophosphate- (cAMP-) and cyclic guanosine monophosphate- (cGMP-) phosphodiesterase activity. It possess anti-inflammatory, bronchodilating properties and used in treatment of asthma. It suppresses the total inflammatory cells, tumor necrosis factor-α (TNF-α) and attenuates the production of interleukins.
3-O-Methylquercetin is a metabolite in flavone and flavonol biosynthesis. It is a naturally occurring compound present in various plants, and was shown to have potent anticancer-promoting, antioxidant, antiallergy, and antimicrobial activity, and showed strong anti-viral activity inhibition of tomato ringspot virus.
Packaging
Bottomless glass bottle. Contents are inside inserted fused cone.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Oral
Storage Class Code
6.1C - Combustible, acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Clement K Ameho et al.
The Journal of nutritional biochemistry, 19(7), 467-474 (2007-10-02)
Dietary antioxidants interact in a dynamic fashion, including recycling and sparing one another, to decrease oxidative stress. Limited information is available regarding the interrelationships in vivo between quercetin and vitamin E. We investigated the antioxidant activity and metabolism of quercetin
C Angeloni et al.
Biochimie, 89(1), 73-82 (2006-10-19)
The aim of this study was to investigate the potential of quercetin and two of its "in vivo" metabolites, 3'-O-methyl quercetin and 4'-O-methyl quercetin, to protect H9c2 cardiomyoblasts against H(2)O(2)-induced oxidative stress. As limited data are available regarding the potential
3-O-methylquercetin more selectively inhibits phosphodiesterase subtype 3
Ko WC, et al.
Planta Medica, 69(04), 310-315 (2003)
Hyang Dok-Go et al.
Brain research, 965(1-2), 130-136 (2003-02-20)
The flavonoids quercetin, (+)-dihydroquercetin, and quercetin 3-methyl ether were isolated from the ethyl acetate fractions of the fruits and stems of Opuntia ficus-indica var. saboten. In the present study, we evaluated their protective effects against oxidative neuronal injuries induced in
Mechanisms of suppression of nitric oxide production by 3-O-methylquercetin in RAW 264.7 cells
Jiang JS, et al.
Journal of Ethnopharmacology, 103(2), 281-287 (2006)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service